Harold Wright
Chief Technologist-UV Disinfection, Carollo Engineers
Andrew Salveson
Water Reuse Practice Director, Carollo Engineers
Bill Sotirakos
Principal UV Technologist, Carollo Engineers
UV technologies play an important role in municipal water, wastewater and water reuse treatment, providing pathogen reduction through disinfection and chemical pollutant reduction through photolysis and advanced oxidation. This article, the second of a two-part series, reviews the applications and regulatory context for UV in municipal water reuse projects in the US, both non-potable and potable applications.
Non-potable reuse disinfection
UV technologies have been used in the US for the disinfection of tertiary wastewater effluents for non-potable reuse applications for almost 30 years. The US EPA published guidelines for water reuse in 1992 specifying that filtered secondary effluents with median fecal coliform counts less than detection can be used for urban reuse, edible crop irrigation and recreational impoundments.
Since 1992, many states have implemented rules governing non-potable reuse. California’s Water Recycling Criteria (WRC, 2000), Title 22, Division 4, Chapter 3, of the California Code of Regulations specifies that filtered secondary effluent that meets 5 log reduction of poliovirus and 2.2 MPN per 100 mL total coliform as a seven-day median can be used for various applications including food crop irrigation, parks, playgrounds, school yards, landscaping, golf courses, recreational impoundments, cooling towers, car washes and artificial snow making. Florida’s Administrative Code (1999) specifies that filtered secondary effluent that has had high-level disinfection, defined as non-detect fecal coliform with 75% of samples, can be used for non-potable reuse.
To support the application of non-potable reuse, the National Water Research Institute (NWRI) in collaboration with the Water Research Foundation (WRF) published the Ultraviolet Disinfection Guidelines for Drinking Water and Water Reuse in 2000. Commonly referred to as the “NWRI UV Guidelines,” the guide was revised in 2003 and 2012, and efforts are currently underway for the next revision, anticipated in 2020. These NWRI UV Guidelines have become, in many locations in the US and around the world, the regulatory guidance tool for non-potable reuse with UV.
The NWRI UV Guidelines are organized as three chapters. Chapter 1 provides guidelines for drinking water applications while Chapter 2 provides guidelines for non-potable reuse. The chapter on drinking water UV disinfection, for the most part, has been superseded in most jurisdictions by the US EPA’s UV Disinfection Guidance Manual (UVDGM 2006) as guidance for drinking water UV applications. The third chapter provides protocols for UV validation, spot-check bioassays, lamp testing and cleaning mechanism testing. The following is an overview of the information in Chapters 2 and 3.
NWRI UV dose requirements
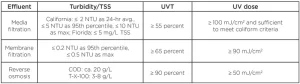
The NWRI UV Guidelines provides water quality criteria and UV dose requirements, defined as MS2 REDs, for three types of filtered secondary effluents, as shown in Table 1. While the guidelines do not identify a target pathogen for reuse applications, they infer that the UV dose requirements are based on achieving 5 log reduction of poliovirus (99.999%) with media and membrane filtered effluents and 3 log reduction with RO effluents, understanding that RO is a robust barrier to virus. The guidelines state that 3 and 5 log reduction of poliovirus occurs with a UV dose of 30 and 50 mJ/cm2, respectively, and that the higher UV dose values of 50, 80 and 100 mJ/cm2 account for “variability in the effluent quality.” Since MS2 phage [dose per log reduction (DL) is ~20 mJ/cm2] is more resistant to UV light than poliovirus (DL = 10 mJ/cm2), the MS2 RED delivered by a UV reactor will be greater than the poliovirus RED. Hence, these higher UV dose values will compensate, to some extent, for these RED bias affects.
The guidelines state that UV systems can be used at UV-Ts that are lower and turbidities that are higher than the criteria provided in Table 1, provided that the selected UV reactor has been validated under those conditions. This is important because many filtered tertiary effluents in places like Florida have UV-Ts that are less than 55%. Regarding TSS and turbidity, it should be recognized that the MS2 RED measured during validation does not depend on turbidity or TSS, and, hence, the issue of elevated turbidity or TSS at a treatment plant cannot be addressed through validation. Instead, it should be addressed by measuring the UV dose-response of the indicator microbes using a collimated beam apparatus.
NWRI UV design criteria
The guidelines state the UV system shall deliver the required UV dose under design criteria of flow, UV-T at 254 nm, lamp output and quartz sleeve fouling. The design flow is defined as the maximum day flow. The design UV-T is defined by the required values listed in Table 1 or the lower 10th percentile of three samples per day measured over a six-month period. The lamp output is conservatively set to 50%, unless the manufacturer conducts lamp testing that justifies a higher value. The fouling factor is set to 80% for manually cleaned systems and conservatively set to 80% with systems that are automatically cleaned unless test data justify a higher value.
Since lamp aging and fouling factors significantly impact the selection of commercial UV systems, manufacturers will often conduct tests under ideal conditions to maximize those values. However, the lamp aging and fouling factors with the installed UV systems (Brooks et al., 2018) are often lower than the values determined using idealized testing. This issue is best addressed using field data collected from several installed systems. For example, the comparison of UV sensor readings in the field to those measured during validation can be used to quantify the combined lamp aging and fouling factor with a given UV system.
NWRI validation
UV systems used for non-potable reuse are typically validated per the NWRI UV Guidelines. The guidelines specify that validation is conducted using MS2 phage as a test microbe. The UV dose-response of MS2 phage should be measured using a collimated beam apparatus equipped with a low-pressure UV lamp. The UV dose delivered by the collimated beam is calculated using:
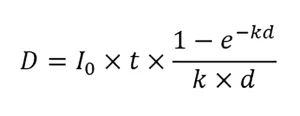
where D is the UV dose, I0 is the incident intensity at the sample surface, t is the exposure time, k is the naperian (base e) UV absorbance coefficient of the water sample at 254 nm, and d is the sample depth. Like the UVDGM, the guidelines state that the value of I0 should be corrected for reflection at the sample surface. However, unlike the UVDGM, the guidelines do not specify that I0 is corrected by the Petri factor and the divergence factor, which can lead to an overestimate of UV dose delivery.
The guidelines specify that the UV dose-response of MS2 phage should be measured from 20 to 150 mJ/cm2 using at least five exposures and fitted using a linear function: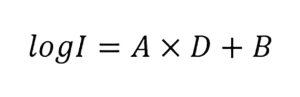
where A and B are constants. As QA/QC, the guidelines state the fit and 80% of the measured UV dose-response data must fall within the bounds defined by:
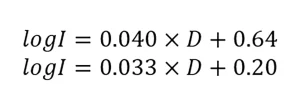
the UV reactor during validation to an MS2 RED. However, because the UV dose-response of MS2 shows curvature, fitting a curved relation with a linear function leads to underestimates of RED at low and high log inactivation and overestimates at intermediate log inactivation.
The guidelines specify that the UV reactors used for postmembrane and RO applications should be validated with drinking water while UV reactors used for post-media filter applications should be validated using filtered reclaimed water with a turbidity greater than 1 NTU. The rationale for this requirement is that particles present in media filtered water impact UV disinfection.
While it is true that wastewater particles can contain microbes, thereby shielding them from UV light, the UV dose-response of MS2 phage added to the flow during validation is unaffected by wastewater particles. For this reason, many manufacturers are conducting UV validation per NWRI UV Guidelines using clean water, and the results are being applied for media filtered, membrane filtered and reverse osmosis effluents.
The guidelines specify that validation testing shall be conducted to demonstrate the impact of flow rate, UV transmittance, ballast power settings and/or UV sensor readings on the UV equipment performance and operational UV dose. If the reactor is configured as banks of lamps in a series, the reactor is tested with various banks operating.
However, the guidelines are not clear on how the validation data are analyzed to define a UV dose algorithm. For example, the guidelines state that the data should be reported as flow per lamp but does not define how that is done with closed vessel and open channel systems. The guidelines also state that REDs can be interpolated as a function of flow, UV-T and UV sensor readings over the tested range but cannot be mathematically assigned to different UV sensor readings or UV-Ts.
While the approach for interpolation of validation data is not defined, the guidelines provide a mathematical example where the RED, determined using the standard MS2 doseresponse relation, is fitted to the log transform of:
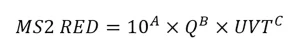
Unlike the UVDGM, the guidelines are not specific on how UV sensor readings are incorporated into the analysis. While some validations have included a term for the relative lamp output calculated using UV sensor equations, similar to what is done with validation conducted in accordance with the US EPA’s UVDGM, others have instead defined RED as a function of ballast power setting and down rated the predicted RED using a lamp aging and fouling factor. This latter approach is problematic when the actual lamp aging and fouling with the UV system with the application is greater than the lamp aging and fouling factor used by the equation. For the most part, recent NWRI validations have defined RED as a function of UV sensor readings as opposed to ballast power level.
The guidelines state that the predicted RED used with the application is defined as the lower 75th percentile prediction interval of the equation fitted to the validation data. The guidelines provide an example where the software XLSTAT is used to calculate the lower 75th percentile MS2 RED with each validation test condition. The ratio of the lower 75th percentile RED to the RED predicted using the regression equation then defines a confidence ratio (CR). The CR value then is used with the equation to define the MS2 RED delivered with the application. For example:
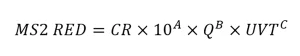
The uncertainty of interpolation used to define the validation factor in the UVDGM is analogous to the confidence ratio specified by the NWRI UV Guidelines.
Spot-check bioassays
The guidelines specify that spot-check bioassays should be used to validate the installed UV system during reactor commissioning. The spot-check bioassay involves at least eight test conditions that include different conditions of flow rate, UV-T, ballast power settings and bank operation.
The guidelines specify that the MS2 RED with seven out of the eight test conditions, calculated using the standard MS2 dose-response curve, should be greater than the lower 75th percentile MS2 RED predicted using the validation. Should more than one of the eight test conditions fail these criteria, the UV reactor’s dose delivery is de-rated based upon the underperforming data.
One challenge with this approach is that equations fitted to validation data can provide biased predictions of UV dose delivery depending on the flow, UV-T and lamp output. Spotcheck tests can therefore be designed to result in optimum results but not necessarily representative results. Future NWRI UV Guidelines should address this challenge by using test conditions that target the UV dose required for the applications.
Summary
The NWRI UV Guidelines have been successfully used for more than a decade to design and validate UV systems for non-potable reuse applications. They have evolved over the years in response to advances in UV dose monitoring and validation and the development of new validation protocols such as the UVDGM. In comparison to the UVDGM, one important benefit of the guidelines is their simplicity for defining UV dose requirements.
There would be value for the industry if there were some harmonization of the UVDGM and the NWRI UV Guidelines, integrating some of the more technical UVDGM aspects without losing the simplicity of the guidelines. Currently, many UV system manufacturers validate their UV reactors with one series of tests and develop a separate UVDGM and NWRI report. While the full validation dataset is used to define the UVDGM equations, only a subset of the MS2 data is used to develop the NWRI equation.
As an alternate approach, all the validation data could be used to define a single equation that predicts log inactivation. That one equation then could be used to predict an NWRI MS2 RED using the standard MS2 dose-response within an NWRI report or could be used to predict a UVDGM RED as needed for pathogen inactivation credit within a UVDGM report.
Because the approach uses the full validation dataset, the resulting analysis would provide a more robust and accurate prediction of NWRI MS2 REDs.
Potable water reuse
Potable water reuse is the purification of municipal wastewater for subsequent use as potable water. For potable water reuse, there are a broad range of chemical and biological constituents that present aesthetic and public health concerns. This review focuses upon the role of UV disinfection and UV advanced oxidation in meeting water quality goals for potable water reuse.
Regulatory guidance and regulations for potable reuse
While there is regulatory guidance for potable water reuse on a state-by-state and national basis (NWRI, 2015; TWDB, 2015; WateReuse Colorado, 2018; NWRI, 2018; NWRI, 2016), the most thorough and formal regulatory effort is from the State of California (DDW, 2018).
While different states have taken different approaches for permitting potable reuse projects, the end goal with pathogen reduction is to achieve less than one in 10,000 annual risk of infection with each examined pathogen group (Regli et al., 1991), a goal which also was used to define Cryptosporidium oocyst reductions as part of the Long Term 2 Enhanced Surface Water Treatment Rule (US EPA, 2006).
This approach provides an additional level of conservatism in comparison to the approach taken with the Surface Water Treatment Rule (SWTR), which defined Giardia goal reduction based on achieving a one in 10,000 annual risk of illness (EPA, 1989).
Table 2 lists the concentrations of Giardia, Cryptosporidium and enteric virus that achieve a one in 10,000 annual risk of infection. To meet the one in 10,000 risk goal, California requires that the treatment train, from the untreated wastewater to the potable water, achieve 12 log removal virus, 10 log removal of Giardia, and 10 log removal of Cryptosporidium (DDW, 2018).

Regarding DDW (2018), two types of potable reuse projects are regulated – groundwater augmentation and reservoir water augmentation. Figure 1 and Figure 2 present schematics of groundwater augmentation and reservoir water augmentation, respectively. Tables 3 and 4 summarize the general requirements for groundwater augmentation and reservoir water augmentation, respectively.
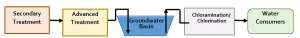

For both groundwater augmentation and reservoir water augmentation, key items pertaining to UV system performance are listed below, with detailed review presented in subsequent sections.
- Robust disinfection of all known pathogens, including virus and protozoa,
- Destruction of NDMA by UV photolysis, and
- Destruction of 1,4-dioxane (as a surrogate) by advanced oxidation (e.g., UV with H2O2) resulting in broad spectrum destruction of other trace level pollutants.
Value of UV to potable reuse
As stated previously, there are three potential applications of UV for potable water reuse, listed here, then detailed with supporting literature below:
- Robust disinfection of all known pathogens, including virus and protozoa. Sizing a UV system for a validated dose of 276 mJ/cm2 results in at least 6 log reduction of a broad range of pathogens, including bacteria, virus and protozoa. It is an important caveat that 6 log reduction by UV requires a particle-free water so that there is no particle shielding of pathogens from UV light.
- Destruction of NDMA by UV photolysis. UV dose values for NDMA destruction should be based upon the feed concentration of NDMA and the target of <10 ng/L. Thus, UV dose values are highly site specific.
- Destruction of 1,4-dioxane (as a surrogate) by advanced oxidation (e.g., UV with H2O2) resulting in broad spectrum destruction of other trace level pollutants. DDW (2018) requires 0.5 log reduction of 1,4-dioxane. The UV dose necessary, combined with an oxidant, is also highly site-specific based upon the level of hydroxyl radical scavengers.
Disinfection
A critical component of designing the treatment and control systems for potable water reuse is a clear understanding of risk and pathogen removal. Quantitative Microbiological Risk Assessment (QMRA) is a powerful tool to analyze risk and optimize treatment and controls for potable water reuse systems (Salveson et al., 2018, Soller et al., 2018, 2017a,b).
The pathogen log reduction by a UV system should be based upon an analysis of risk and an understanding of the pathogen removal capabilities of the other processes in the treatment train. DDW (2018) requires that potable reuse projects for groundwater recharge provide a combined level of treatment resulting in 12 log virus reduction, 10 log Giardia reduction and 10 log Cryptosporidium reduction (12/10/10-log removal). No single process used by the treatment train can claim more than 6 log reduction credit. DDW (2018) also states that at least three processes must provide at least 1 log reduction. Beyond those three key processes, processes that provide <1 log reduction can be included within the analysis. UV disinfection is often used to achieve 6 log reduction.
For potable reuse applications using UV, disinfection credit should be based upon adenovirus disinfection. Adenoviruses comprise a large group of serologically different viruses that can cause a broad spectrum of diseases with varying severity (US EPA, 2010). The UV dose required for 6 log inactivation of adenovirus, determined using a collimated beam apparatus equipped with a low-pressure UV lamp, is 276 mJ/cm2 (US EPA, 2018).
UV photolysis of NDMA
The UV dose necessary for NDMA destruction is dependent upon the feed concentrations of NDMA. As one practical example, Oxnard (2018) is cited.
Oxnard’s advanced water treatment system for potable water reuse employs a high-dose UV system after reverse osmosis (RO).
NDMA concentrations in the RO permeate range from 11 ng/L to 32 ng/L. As a measure of conservatism and targeting a UV effluent NDMA value of 5 ng/L (which is 50% of the California Notification Level for NDMA), Oxnard has adopted a goal of 0.8 log reduction of NDMA through high-dose UV photolysis. The next question then becomes, what UV dose? As detailed in Oxnard (2018), the literature reports that 1 log reduction of NDMA requires a UV dose ranging from 700 to 1,100 mJ/cm2 (Health Canada, 2011). Based upon the need for 0.8 log reduction of NDMA, a UV dose of 800 mJ/cm2 was selected for UV operation. Importantly, after accounting for RED bias effects and the uncertainty of validation per the UVDGM, the UV dose for NDMA reduction is greater than the validated dose of 276 mJ/cm2 necessary for 6 log reduction of pathogens.
UV advanced oxidation process (UV AOP)
To be clear, only the state of California currently requires UV AOP for the destruction of 1,4-dioxane for potable reuse projects involving groundwater and reservoir water augmentation. UV AOP should be viewed as a safety factor employed on top of existing robust treatment. Detailed analysis of 1,4-dioxane destruction via UV with H2O2 was documented by Oxnard (2018).
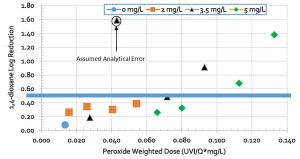
Results showed a relation between peroxide-weighted UV dose and 1,4-dioxane destruction; as shown in Figure 3. UV dose is expressed as UV intensity (UV-I) divided by flow (Q). For Oxnard (2018), the approximate UV dose of 1,000 mJ/cm2 and an H2O2 dose of 6 mg/L was determined to be sufficient to meet the DDW (2018) requirements for 0.5 log reduction of 1,4-dioxane.

Advanced oxidation processes (AOP) are highly sensitive to the presence of hydroxyl radical scavengers, such as TOC, alkalinity, chloramines, bromide and nitrite. RO permeate has relatively low levels of scavengers, allowing for the UV AOP to be effective. IDE Technologies (IDE, 2019), as part of extensive potable reuse pilot testing in Pismo Beach, California, closely examined the UV AOP performance with different water qualities using a UV reactor with electrodes (instead of H2O2) to destroy 1,4-dioxane (Figure 4).
Figure 5 demonstrates the results with and without chloramines, which are a known and substantial scavenger of hydroxyl radicals. For the case of
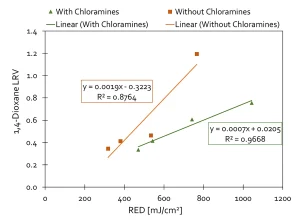
Pismo Beach, the UV dose required to destroy 0.5 log of 1,4-dioxane varied from 500 to over 800 mJ/cm2, depending upon chloramine concentration.
Summary of UV for potable reuse
UV, due in particular to its robust pathogen reduction at high dose values, is a critical component of the pathogen treatment barrier for potable reuse systems. Further, the photolysis and advanced oxidation ability of UV, for some potable reuse applications, is an important chemical treatment barrier.
Contact: Harold Wright, hwright@carollo.com; Andrew Salveson, asalveson@carollo.com; Bill Sotirakos, bsotirakos@carollo.com
References
Brooks, T., Wright, H., Heath, M. and Salveson, A. (2018) Do’s and Don’ts of Wastewater and Reuse UV System Implementation. PNCWA Annual Conference, October 231-24, Boise, ID.
DDW (2018). California Regulations Related to Recycled Water. California Code of Regulation Title 22, Division 4, Chapter 3. Published October 8, 2018.
Haas, C., Crockett, C., Rose, J., Gerba, C., and Fazil, A. (1996). Assessing the risk posed by oocysts in drinking water. Journal AWWA, September 1996.
Health Canada (2011). Guidelines for Canadian Drinking Water Quality: Guideline Technical Document: N-Nitrosodimethylamine (NDMA). Ottawa, Ontario.
IDE (2019). IDE Technologies Central Coast Blue Demonstration Report. A report by Carollo Engineers, Inc., WSC, and the City of Pismo Beach. May 2019.
NWRI (2015). Framework for Direct Potable Reuse. A publication by the National Water Research Institute in collaboration with the WateReuse Association, The American Water Works Association, and the Water Environment Federation. Alexandria, Virginia.
NWRI (2016). Final Report of an NWRI Independent Advisory Panel: Recommended DPR General Guidelines and Operational Requirements for New Mexico. Prepared by NWRI for the New Mexico Environment Department.
NWRI (2018). Guidance Framework for Direct Potable Reuse in Arizona. Prepared by NWRI for WateReuse Arizona, the AZ Water Association, and the Steering Committee for Arizona Potable Reuse.
Oxnard (2018). Engineering Report for Groundwater Replenishment Reuse Project. December 2018. A report by Carollo Engineers, Inc.
Regli, S., J.B. Rose, C.N. Haas, and C.P. Gerba (1991). “Modeling the Risk from Giardia and Viruses in Drinking Water.” JAWWA, November 1991, 76-84.
Salveson, A., Dickenson, E., Soller, J., Angelotti, B., Parker, A. (2018). Pathogen Risk Evaluation of Treatment and Monitoring System Performance for Potable Reuse. Water Research Foundation Project 4767. Denver Colorado.
Soller, J., Parker, A., Salveson, A. (2018). Public Health Implications for Short Duration, Off-Specification Conditions at Potable Water Reuse Water Treatment Facilities. Environ.Sci.Technol. Lett. 2018, 5, 675-680.
Soller, J.A., Schoen, M., Steele, J.A., Griffith, J.F. and Schiff, K.C. (2017a) Incidence of gastrointestinal illness following wet weather recreational exposures: Harmonization of quantitative microbial risk assessment with an epidemiologic investigation of surfers. Water Res 121, 280-289.
Soller, J., Eftim, S., Warren, O., Nappier, S. (2017b) Evaluation of Microbiological Risks Associated with Direct Potable Reuse. Microbial Risk Analysis 5 (2017) 3-14.
Texas Water Development Board (TWDB, 2015). Direct Potable Reuse Resource Document. Final Report. Prepared by Alan Plummer Associations, Inc. for the Texas Water Development Board.
Trussell, R.R., A. Salveson, S.A. Snyder, R.S. Trussell, D. Gerrity, and B. Pecson (2013). “Potable Reuse: State of the Science Report and Equivalency Criteria for Treatment Trains,” a Report for WateReuse Research Foundation Project 11-02, Alexandria, VA.
USEPA (1989). Surface Water Treatment Rule. 40 CFR Parts 141 and 142 Part III. Federal Register, Vol. 54, No. 124, pages 27,544-27,568, June 29, 1989.
USEPA (2006). Long Term 2 Enhanced Surface Water Treatment Rule. 71 CFR page 654, Federal Register, January 5.
USEPA (2010). “Water Research Foundation (WRF), Challenge Organisms for Inactivation of Viruses by Ultraviolet Treatment.” 2010.
USEPA (2018). Innovative Approaches for Validation of Ultraviolet Disinfection Reactors for Drinking Water Systems. Draft final. October 2018.
Verhoeven, S., L. Sealey, L., J. Patterson, J. and C. Odegaard, C. (2017). Not All all Water water Is is Created created Equalequal: The Effects effects of Water water Characteristics characteristics on MS2 Stabilitystability. Presented at the 2017 IUVA Americas Conference, February 5-8, Austin, Texas.
WateReuse Colorado (2018). Technical Memorandum 1: Development of DPR Regulations in Colorado. Prepared by Carollo Engineers, Inc. for WateReuse Colorado.
For part 1 of this series, view this article at www.uvsolutionsmag.com.