By Troy Cowan, IUVA Healthcare/UV Working Group Facilitator
COVID-19 is worldwide, mutating and becoming more infectious with each new variant, such as Omicron (Variant BA.1) and its “sister” (Variant BA.2), spreading several times faster than the original or the Delta strains.1 As highlighted in a recent World Health Organization (WHO) webinar, COVID-19 is spread from anyone who’s contagious to healthy people just by being in the same room.2 A recent Time magazine article reminds us that “COVID-variants may be with us for years to come, and this will certainly not be the last respiratory virus pandemic.”3 The Time article then makes a powerful, thought-provoking statement – “…we need to think clearly and scientifically about how better we can reduce the spread of viruses indoors especially when and where masks will no longer be in common use.”4
Since 1942, it has been well documented that ultraviolet irradiation is a viable and effective preventative tool in the fight against Tuberculosis and other pathogenic microorganisms.5 Additional evidence comes from the following:
- The WHO webinar, documenting ventilation systems as powerful tools in reducing pathogen loads from infected people in the room – and UV-C, when applied via upper-air or whole-room devices designed to combat airborne pathogens, can further improve these reductions by several factors of 10.6
- CDC’s guidance for “Ventilation in Buildings,” where it even mentions using “ultraviolet germicidal irradiation (UVGI) as a supplemental treatment to inactivate SARS-CoV-2…”7
That said, it should be noted that getting updated information from the CDC is difficult. The CDC webpage shown for UVGI is noted as “archived,” only available for “historic purposes, last updated in April 2021.”8 Many advances have been made in researching the effectiveness of UV-C against COVID related pathogens since then that have not been recognized by the CDC. For example:
- Several recent findings showing “UV-C rapidly inactivates SARS-CoV-2, the virus that causes COVID-19, as well as other common airborne pathogens such as influenza viruses.”9
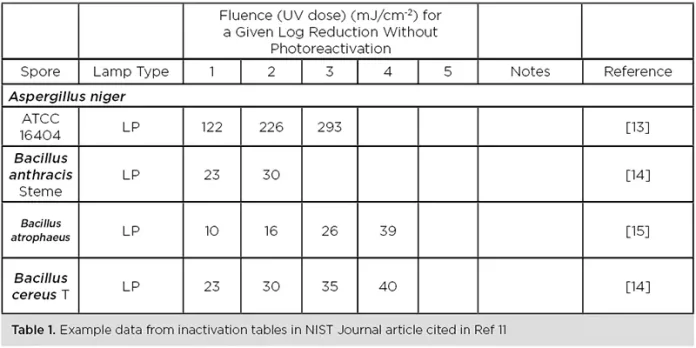
- The National Institute of Standards and Technology (NIST) releasing its Journal of Research Special Section on Ultraviolet Technologies for Public Health in August 2021, with more than a dozen peer-reviewed papers on UV-C disinfection technology.10 This includes a compilation of inactivation data on more than 2,000 pathogens from 250+ peer-reviewed journals (Table 1).11
- The American Conference of Governmental Industrial Hygienists’ releasing updated Threshold Limit Values from Chemical Substances and Physical Agents & Biological Exposure Indices, which revised Threshold Limit Values (TLVs®) for UV-C (200 nm to 280 nm), and Far UV-C (200 nm to 235 nm), showing they have a wider margin of safety than previously thought, and that Far UV-C even may be cleared for routine direct human exposure without concern for injury.12
These are significant developments that directly address how UV-C can help make “life after masks” safer and more livable, especially in public spaces – and not only for COVID-19, but also safer against other pathogens spread by respiratory droplets, such as tuberculosis, varicella (virus which causes chickenpox), measles and even influenza. Why are they not being addressed?
Turning from healthcare to the regulatory side, the EPA is getting more involved in regulatory enforcement, especially when it comes to “false and misleading” labels, as reported last quarter.13 Many of EPA’s pronouncements appear to be based on erroneous assumptions and even some misunderstanding of UV-C technology and science. This becomes apparent when we analyze EPA’s April 12, 2021, letter to the National Electrical Manufacturers Association14 and its implications, where:
- EPA states that it “assesses claims (of efficacy) on a case-by-case basis,” yet it “does not routinely review the safety or efficacy of pesticidal devices…, including UV light products, and cannot confirm whether – or under what circumstances – such products might be effective.” If the EPA does not review efficacy (as it says) and cannot confirm a product might be effective, how can it credibly assess a claim of efficacy?
- EPA maintains that “testing of the device against those pathogens on the specific substrate (e.g., E. coli on cloth) is necessary to substantiate those claims.”15 Why does the EPA not use the more than 250 peer-reviewed studies and data sets on the UV dosages required to inactivate more than 2,000 pathogens that can be used to substantiate the basics of such claims? Why does the EPA not acknowledge that testing of UV on various pathogens varies dramatically from testing of pesticides, in that it relies only on the delivered photonic energy to produce its effects, and this does not vary significantly due to the underlying substrates, as “the biological effect of light energy is directly related to the total energy or dose delivered, irrespective of how it is administered,” per the Bunsen-Roscoe Law, a fundamental law of photobiology dating back to the 19th century?16
- EPA asserts that a simple claim of being “germicidal lights” is on its face “false and misleading” unless supported by efficacy testing. Why doesn’t the EPA acknowledge that any UV radiation (especially UV-C) already is federally recognized by FDA as a known disinfectant,17 and is therefore, by definition, “germicidal” (i.e., “something [such as an antiseptic or disinfectant] that destroys or inactivates pathogens”18), without regard to an efficacy test?
While developing the above analysis, this author happened across an article in a recent nuclear industry newsletter and was very surprised by how our two very different industries both are similarly constrained by “[a]n antiquated regulatory system coupled with regulators’ lack of understanding of advanced … technology is a barrier to the deployment of next-generation [technologies]…”19
In an effort to “break free” from these barriers, we are working in collaboration with key industry standards development organizations (SDOs) to develop ANSI-certified standards that will have industry-wide impact and meet the Federal definition of “voluntary consensus standards.”20 By doing this, we can invoke OMB Circular A-119 “Federal Participation in the Development and Use of Voluntary Consensus Standards and in Conformity Assessment Activities,” and alleviate the need for a Federal agency to impose Federal regulations covering the same or similar requirements covered by ANSI standards.21
Specifically, OMB A-119 specifies that “…all Federal agencies must use voluntary consensus standards in lieu of government-unique standards in their procurement and regulatory activities except where inconsistent with law or otherwise impractical.”22 Thus, if industry standards exist, it eliminates the need for and cost of developing overlapping Federal standards, decreases the cost of goods procured and the burden of complying with agency regulation, while assisting the Federal agencies in meeting their obligation to reducing regulatory requirements.23, 24
With this in mind, the UV industry has several ANSI-related standards initiatives underway to put industry voluntary consensus standards in place, covering topics essential to measuring UV performance in a repeatable, auditable manner. These include the following, with more to follow:
- UV Output Measurement Standards: Working together, IES and IUVA are developing standards for measuring UV output in a standardized and auditable manner – BSR/IES LM-91 (C303)-202x (“Application Distance Specific Radiometry”) and BSR/IES/IUVA LM-92-202x (“Optical and Electrical Measurement of Ultraviolet LEDs”). Both are going through the ANSI review process,25 expected to be published in March. Additional standards are in process and being proposed, e.g., LM-93 covering Far UV-C measurement, now being drafted in committee, and a proposal for standards related to calibrating dosimeters and radiometers used for these measurements.
- UV Whole Room and HVAC Disinfection Standards: IUVA is working collaboratively with ASHRAE, through its SDO process, on SPC 185.3, covering generic air disinfection (applicable to any technology) and STPC 185.4 on surface inactivation and room disinfection. Both are in the initial stages of development, with final review and approval schedules to be determined.
- Labeling of UV-C Products: IUVA has initiated efforts to draft a voluntary labeling and product information template for various UV-C devices and key components (e.g., lamps) as a way to uniformly and clearly present information on the product, its configuration, intended uses and performance, as measured in accordance with applicable industry standards (e.g., BSR/IES LM-91, SPC 185.3, noted above, once approved). In this way, it would capture industry-developed guidelines for label content that could be developed into voluntary consensus standards, as a way to promote positive, informative labeling and advertising guidance. If adopted by an SDO and nationally certified, per in OMB A-119,26 it could be used to provide better definition and boundaries for when something should or should not be deemed “false and misleading” for purposes of EPA regulatory compliance.
In summary, we will continue to work with our existing industry partners (i.e., IES, ASHRAE) and continue to reach out to others (e.g., NEMA, GLA) to identify, develop and implement the industry voluntary consensus standards necessary to fill known gaps in existing guidance. Others we know are needed include a comprehensive UV lexicon and a protocol for collecting and analyzing UV inactivation fluence values by pathogen in a reproducible and auditable manner. The goal is to have credible industry standards in place that assure the healthcare sector and public consumers that they are getting the UV device information they need to make credible, science-based decisions, and the information they’ve been provided is useful, accurate and valid.
By its nature, this has to be a very collaborative and inclusive process if we’re to be ready for the next pandemic with suitable and useful UV technologies. Again, per the Time article, “…we need to think clearly and scientifically about how better we can reduce the spread of viruses indoors especially when and where masks will no longer be in common use.” UV can be a significant part of that solution if we move now. We welcome participation from the UV industry, from the healthcare sector and from our Federal regulators in making this happen.
It’s all about saving lives.
The IUVA Healthcare/UV Working Group endeavors to promote the acceptance of UV disinfecting technologies as a credible, valued part of environmental management throughout the healthcare industry. In this column, the UV community will be updated on these efforts and the latest information on UV technology as it pertains to the healthcare industry.
Contact: Troy Cowan, troy@visionbasedconsulting.us
References
- Lauren Pelley, “Omicron subvariant BA.2 raises new questions about puzzling evolution of virus behind COVID-19,” CBC News posted: Jan 26, 2022 4:00 AM ET | Last Updated: January 26 (https://www.cbc.ca/news/health/omicron-subvariant-ba-2-raises-new-questions-about-puzzling-evolution-of-virus-behind-covid-19-1.6327270), last accessed 8 Feb. 2022
- Prof. E. Nardell, “An Overview of UV-C Disinfection,” part of the World Health Organization Webinar “Disinfection using Ultraviolet Radiation and its relevance to COVID-19 management,” 17 Dec. 2021, https://cdn.who.int/media/docs/default-source/radiation/webinar-uv-disinfection-dec-2021.pdf?sfvrsn=4bcda684_5), last accessed 13 Feb. 2022
- Prof. E. Nardell, “If We’re Going to Live With COVID-19, It’s Time to Clean Our Indoor Air Properly,” Time Magazine, 1 February 2022, (https://time.com/6143799/covid-19-indoor-air-cleaning/) last accessed 8 Feb. 2022
- Ibid.
- CDC/NIOSH, “Environmental Control for Tuberculosis: Basic Upper-Room Ultraviolet Germicidal Irradiation Guidelines for Healthcare Settings,” DHHS (NIOSH) Publication No. 2009–105, March 2009
- Nardell, WHO Webinar, cited above
- CDC, “Ventilation in Buildings,” updated June 2, 2021 (https://www.cdc.gov/coronavirus/2019-ncov/community/ventilation.html), last accessed 8 Feb. 2022
- CDC, Archived Webpage “Upper-Room Ultraviolet Germicidal Irradiation (UVGI)”, updated 9 April 2021 (https://www.cdc.gov/coronavirus/2019-ncov/community/ventilation/UVGI.html, last accessed 8 Feb. 2022
- IUVA Task Force on Far UV-C Radiation for Disinfection of Air and Surfaces (Blatchley, et al.), “Far UV-C Radiation: Current State-of Knowledge,” updated May 2021, (https://iuva.org/resources/covid-19/Far%20UV-C%20Radiation-%20Current%20State-of%20Knowledge.pdf), last referenced 8 Feb 2022.
- National Institute of Standards and Technology (NIST) Special Section on Ultraviolet Technologies for Public Health, Volume 126 (2021), ISSN: 2165-7254, (https://www.nist.gov/nist-research-library/journal-research-nist), last accessed 8 Feb 2022
- Masjoudi, Mohseni, and Bolton, “Sensitivity of Bacteria, Protozoa, Viruses, and Other Microorganisms to Ultraviolet Radiation,” Journal of Research of the National Institute of Standards and Technology, Volume 126, Article No. 126021 (20 August 2021), (https://doi.org/10.6028/jres.126.021), last accessed 8 Feb 2022
- American Conference of Governmental Industrial Hygienists, “2022 Threshold Limit Values from Chemical Substances and Physical Agents & Biological Exposure Indices,” ACGIH, Cincinnati, 2022.
- R. Zarghamee, et al., “EPA Takes Aim at Purification Devices,” UV Solutions, 9 Dec. 2021 (https://uvsolutionsmag.com/articles/2021/epa-takes-aim-at-purification-devices/), last accessed 8 Feb 2022
- Memorandum from Pease and Segall (EPA) to Tolsdorf (NEMA), 12 April 2021 (https://www.epa.gov/sites/default/files/2021-05/documents/nema-response_4-12-2021.pdf) last accessed 13 Feb 2022
- Masjoudi, Mohseni, and Bolton, cited above, last accessed 8 Feb 2022
- Kumar, Peña, Willner, and Kreitenberg, “UV-C Intensity, Time and Total Dose Germicidal Efficacy,” UV Solutions, 9 Dec 2021, (https://uvsolutionsmag.com/articles/2021/uv-c-intensity-time-and-total-dose-germicidal-efficacy/) last accessed 8 Feb 2022
- FDA, “UV Lights and Lamps: Ultraviolet-C Radiation, Disinfection, and Coronavirus,” webpage, 1 Feb 2021, (https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/uv-lights-and-lamps-ultraviolet-c-radiation-disinfection-and-coronavirus), last accessed 8 Feb 2022
- Merriam-Webster, definition of “germicide,” (https://www.merriam-webster.com/dictionary/germicide), last accessed 8 Feb 2021
- American Nuclear Society, “Outdated regulations slowing advanced reactor progress,” ANS Nuclear SmartBrief email, February 7, 2022, (https://www.smartbrief.com/uyd/editProfile.action;jsessionid=20E318356CCE83334B0182AA48C6FA1E.web2.smartbrief.com?t1=71903157&t2=https%3A%2F%2Fwww.bloomberg.com%2Fnews%2Farticles%2F2022-02-07%2Fnuclear-industry-says-government-regulators-don-t-understand-new-small-reactors), last accessed February 7, 2022
- OMB A-119, “Federal Participation in the Development and Use of Voluntary Consensus Standards and in Conformity Assessment Activities,” Item 5 “What is the Policy for Federal Use of Standards?,” https://www.whitehouse.gov/wp-content/uploads/2020/07/revised_circular_a-119_as_of_1_22.pdf, last accessed Feb 6, 2022)
- Ibid.
- Ibid.
- OMB, “Improving Regulation and Regulatory Review,” Executive Order 13563 of January 18, 2011 (https://obamawhitehouse.archives.gov/sites/default/files/omb/inforeg/eo12866/eo13563_01182011.pdf), last accessed 13 Feb 2022
- OMB, “Identifying and Reducing Regulatory Burdens,” Executive Order 13610 of May 10, 2012 (https://obamawhitehouse.archives.gov/sites/default/files/docs/microsites/omb/eo_13610_identifying_and_reducing_regulatory_burdens.pdf), last accessed 13 Feb 2022
- American National Standards Institute, “ANSI Standards Action Bulletin,” Vol. 5, No. 51, 17 Dec. 2021, pg. 22, (https://standards.incits.org/apps/group_public/download.php/137014/livelink), last accessed 13 Feb. 2022
- OMB A-119, Item 5, cited above