Kyle D. Rauch, Stephanie Gora, Carolina Ontiveros and Graham Gagnon
Centre for Water Resource Studies, Dalhousie University, Halifax, NS, Canada
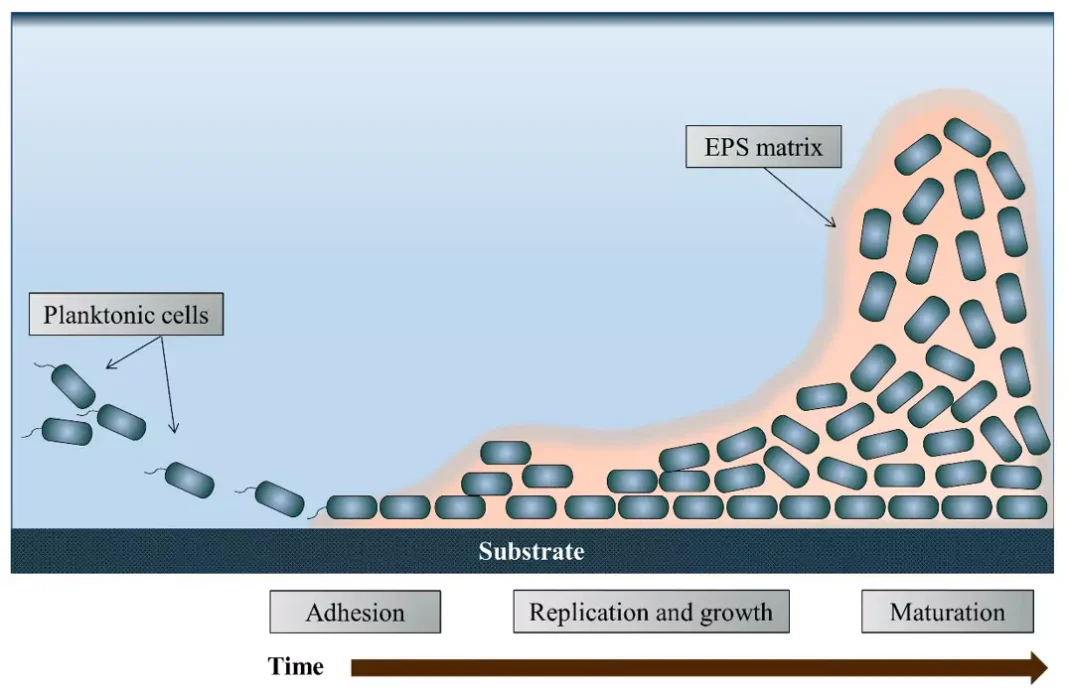
Biofilms are ubiquitous in nature and have the potential to harbor dangerous, opportunistic pathogens. Biofilms form when planktonic bacteria come into contact with a wetted surface and begin to release extracellular polymeric substances (EPS), allowing them to adhere to the solid substrate (Figure 1). Once the bacteria have adhered, they begin to replicate and increase EPS production, thickening the layer of the biofilm and securing the structure to the substrate.
The EPS matrix provides protection against common disinfection methods and makes disinfection of microorganisms encased in the biofilm increasingly difficult. This increased challenge to disinfection becomes even more problematic when opportunistic pathogens (e.g. Pseudomonas aeruginosa and Legionella pneumophila) find refuge in the biofilms. Infections acquired from biofilm-bound bacteria can cause severe illness and even death in individuals with compromised immune systems.1 Biofilm-bound bacteria have been associated with 80% of bacterial infections in the US2 and are a common concern for food and healthcare industries.
In a clinical setting, biofilms can contaminate a wide range of infrastructure, tools and devices including, but not limited to, showerheads, hot water storage, dental water lines, endoscopes, catheters, pacemakers and prosthetic heart valves etc.3 P. aeruginosa infections acquired in the clinical setting have been shown to increase the rate of illness and death rates of patients.4,5
In the food industry, meat, dairy, fish, poultry and produce are all at risk of biofilm contamination that can arise at any point from farming to consumption. Major risks of contamination stem from the product coming into contact with tainted process water, work surfaces and equipment.2 Contamination of food products can ultimately cause serious health consequences if the food is consumed and also can cause financial losses for a company through increased spoilage and loss of product.3
Biofilms are also a common concern in the water industry, as they can form on water treatment process equipment (e.g. membrane filters), distribution system infrastructure, premise plumbing, water storage tanks and secondary storage containers. The results of previous studies suggest that opportunistic pathogens, including Legionella, can grow inside of biofilm-dwelling hosts (e.g. protozoa) in drinking water infrastructure.6 These risks often are exacerbated in remote and/or decentralized applications where water treatment technology is limited and the maintenance of onsite drinking water systems is the responsibility of residents or building owners with minimal training (e.g. Farenhorst et al.7).
In the food, healthcare and water sectors, many biofilm control strategies have been implemented, including chemical control with such agents as sodium hypochlorite, hydrogen peroxide, peracetic acid and ozone2; however, introduction to chemical agents eventually may lead to the development of resistant microbial populations.8,9 Other types of control methods – such as ultrasonication, phages, enzymatic solutions, UV/H2O2 and UV/Cl2 – also have been employed to mitigate biofilms.2
UV irradiation alone also has been studied by many researchers with varying results.10,11 The different methods employed by the researchers applying UV irradiation in different niche applications may have given rise to the inconsistent results. However, common across these studies is the finding that much higher UV fluences are required to achieve similar levels of inactivation of biofilm-bound microorganisms compared to planktonic (free floating) microorganisms.10,12,13
One study has shown that this is likely due to the high cellular density within the EPS matrix, which leads to increased attenuation of the UV light through the depth of the biofilm.10 Furthermore, the complex EPS matrix also may provide shielding effects.14
A previous study suggests that a control strategy that first disrupts or dissolves the EPS matrix using surfactant should be adopted, as it will increase the penetration of chemical disinfectants.15 A similar method potentially could be employed to allow for better penetration of UV-C irradiation into biofilms.
The objectives of this work were to develop a standard protocol for inactivating P. aeruginosa in biofilms with a UV-C LED collimated beam apparatus, compare inactivation from UV-C irradiation to commercial disinfecting wipes and investigate the impacts that the application of commercial disinfecting wipes before UV-C irradiation have on inactivation.
UV-C LED method development
The methodologies employed in previous studies focused on the application of UV for biofilm mitigation reflecting the industry-specific questions being addressed by the researchers who developed the experiments and apparatus. As a result, it is difficult to compare and generalize the results of these studies. A robust and repeatable method was developed for growing, treating and recovering biofilms for UV-C LED inactivation experiments. A full description of this standardized method can be found in Gora, et al.16
A CDC biofilm reactor and the method developed by the US EPA17 were employed to grow stable P. aeruginosa (PA01) biofilms on polycarbonate coupons. The growth process included two stages designed to encourage biofilm adhesion and maturation. In the first stage, 500 mL of nutrient-rich media was inoculated with 1 mL of a highly concentrated P. aeruginosa stock and maintained at room temperature for 24 hours. This stage ensured the bacteria adhered to the surface of the coupon and initialized biofilm formation.
In the second stage, the nutrient-rich media was fed slowly into the reactor at approximately 10 mL/min at room temperature for an additional 24 hours. This stage allowed the attached biofilm to develop and mature.
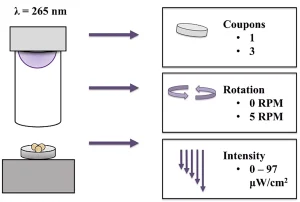
Next, treatment was optimized with the UV-C LED collimated beam apparatus (Aquisense Technologies, Kentucky, US) based on three factors: the number of coupons being treated, rotation vs. no rotation and UV intensity (Figure 2). The study found that increased inactivation was achieved when samples were rotated at 5 RPM and that the number of coupons and intensity of the UV-C LEDs had no impact on inactivation. Thus, it was decided to treat three coupons at a time with a rotation of 5 RPM at the highest possible intensity for the remainder of the study.
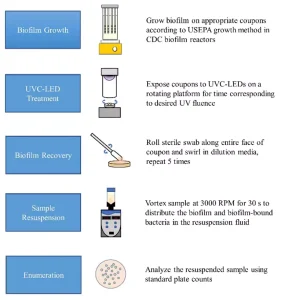
Scraping and swabbing were compared as biofilm recovery methods and hand mixing, vortexing, sonicating and stomaching as biofilm resuspension methods. The materials and settings used for the different recovery methods were adapted from a previous study examining efficient biofilm removal from annular reactor coupons.18
The resuspended samples were enumerated on tryptic soy agar (TSA) plates at 37°C for 18 to 24 hours. We determined the combination of recovery and resuspension methods that gave the highest yield of P. aeruginosa cells and used it in the remainder of the study. A summary of the recommended standard method for UV-C LED treatment of P. aeruginosa biofilms is outlined in Figure 3.
Experimental
The 265 nm UV-C LED used in this study was characterized and found to have a peak wavelength of 268 nm and an FWHM of 11.5 nm. The intensity of the UV-C light delivered to the samples was determined by measuring the average intensity over the treatment field of the three coupons with a USB4000 spectrometer equipped with a DET4-200-850 detector (Ocean Optics Inc., Florida, US).
Intensity measurements were collected with a 0.5 cm spatial resolution at the surface of the coupons. The total intensity under the spectrum of the UV-C LED was collected by integrating the peak from 220 nm to 300 nm for each point in the treatment field. Once the average intensity was measured, the exposure times required to achieve UV fluences ranging from 0 to 12 mJ/cm2 were calculated by dividing the required fluence by the average intensity.
P. aeruginosa biofilms were grown on polycarbonate coupons in a CDC biofilm reactor using the above method. Following growth, treatment with UV-C LED irradiation, common disinfectant wipes or a combination of wiping followed by UV-C LED irradiation was applied to the contaminated coupons. UV fluences between 0 and 60 mJ/cm2 were applied using a UV-C LED collimated beam apparatus (Aquisense Technologies).
For the wiping treatments, a conventional disinfecting wipe (Cavi-Wipes, Metrex; Orange, California, US) were used in either a single pass or contacted with the surface for 15 seconds. Isopropyl alcohol (17.2%) and ammonium chloride (0.28%) are the active ingredients in Cavi-Wipes.
Results and discussion
Inactivation from UV treatment alone was shown to plateau at approximately 1.3-log reduction after a UV fluence of 8 mJ/cm2 (data not shown, see publication for kinetics16). This was not entirely unexpected, as the inactivation of biofilm-bound microorganisms has been shown to require significantly higher fluences compared to free floating planktonic cells.
For example, research has shown that a fluence of approximately 4 mJ/cm2 can achieve a similar log inactivation of 2 log in a pure suspension of planktonic P. aeruginosa.19
When examining indigenous bacterial communities in catheters, it was shown that the UV fluence required to achieve 4 log inactivation in the biofilm community was 10 times greater than that of the resuspended biofilm.10 In another study, authors examined UV-C LED inactivation of P. aeruginosa in biofilms formed on catheters and found that a 4-log inactivation could be achieved with a fluence of 7.9 mJ/cm2.20
Treatment with commercial disinfecting wipes alone resulted in minimal differences between the single pass and the 15-second contact time and saw a slightly increased disinfection potential when compared to UV-C LED treatment alone.
However, when used in combination with UV-C LED irradiation, synergistic effects were observed. For example, a 2.3-log reduction was achieved with a single pass of the commercial disinfecting wipe, and when a 12 mJ/cm2 fluence was applied to the biofilm a 1.3-log reduction was achieved.
When the two methods were used in combination, the resulting plates contained too few colonies to count, indicating that more than 7.8-log inactivation was achieved. This suggests that up to 4.2-log inactivation was potentially related to synergistic effects between the wiping and UV-C LED treatments.
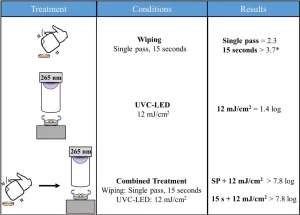
It was hypothesized that these effects may have occurred because the wiping treatment disrupted the biofilm and decreased any shielding effect the EPS matrix may have been providing to the cells (see Figure 4).
Interactions between the residual chemicals from the wipes and UV-C irradiation also may have contributed to the improvement in inactivation. The mechanism(s) underlying the synergistic effects reported here is the focus of current research into biofilm prevention and mitigation on surfaces.
This study demonstrated that the effectiveness of treating polycarbonate surfaces contaminated with P. aeruginosa biofilms was greatly improved by wiping with commercial disinfecting wipes followed by UV-C LED irradiation.
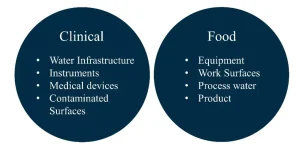
While these findings may not be appropriate for all the areas where biofilm control strategies may be required (Figure 5), it is believed that the protocol presented will allow researchers to examine how factors such as active ingredients in disinfecting wipes, substrate materials, bacterial species and UV wavelengths impact the inactivation of biofilm-bound microorganisms.
Conclusion
This study examined the best method to systemically treat P. aeruginosa biofilms grown on polycarbonate coupons with a UV-C LED collimated beam apparatus. The method developed from this work provides a robust, bench-scale protocol to investigate inactivation responses of biofilm-bound bacteria that could be adapted for other industry-specific needs. This study also investigated the effectiveness of commonly used isopropanol disinfectant wipes on biofilm mitigation and the impacts of combing the wipes with UV-C LED irradiation.
When the two methods were combined, a 7.9-log reduction in P. aeruginosa was achieved, well above the reductions observed for either treatment alone. The mechanisms behind these synergistic effects were not explored in this study, but it is hypothesized that the increased reduction in cells was likely due to mechanical and/or chemical disruption of the EPS matrix prior to the application of UV-C irradiation. This hypothesis is currently being tested in the laboratory. The findings from this study suggest a two-stage treatment approach combining disinfectant wipes and UV-C LED irradiation could prove to be highly effective for biofilm control on surfaces.
Contact: Kyle D. Rauch, kyle.d.rauch@dal.ca; Stephanie Gora, stephanie.gora@dal.ca; Carolina Ontiveros, carolina.ontiveros@dal.ca; Graham Gagnon, graham.gagnon@dal.ca
References
- Kerr KG, Snelling AM. Pseudomonas aeruginosa : a formidable and ever-present adversary. J Hosp Infect. 2009;73(4):338-344. doi:10.1016/j.jhin.2009.04.020
- Srey S, Jahid IK, Ha S. Biofilm formation in food industries : A food safety concern. Food Control. 2013;31(2):572-585. doi:10.1016/j.foodcont.2012.12.001
- Lindsay D, von Holy A. Bacterial biofilms within the clinical setting: what healthcare professionals should know. J Hosp Infect. 2006;64(4):313-325. doi:10.1016/j.jhin.2006.06.028
- Osmon S, Ward S, Fraser VJ, Kollef MH. Hospital Mortality for Patients with Bacteremia Due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest. 2004;125(2):607-616. doi:10.1378/chest.125.2.607
- Rello J, Rue M, Jubert P, et al. Survival in patients with nosocomial pneumonia: Impact of the severity of illness and the etiologic agent. Crit Care Med. 1997;25(11):1862-1867.
- Falkinham JO, Pruden A, Edwards M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. 2015:373-386. doi:10.3390/pathogens4020373
- Farenhorst A, Li R, Jahan M, et al. Bacteria in drinking water sources of a First Nation reserve in Canada. Sci Total Environ. 2017;575:813-819. doi:10.1016/j.scitotenv.2016.09.138
- Tattawasart U, Maillard JY, Furr JR, Russell AD. Development of resistance to chlorhexidine diacetate and cetylpyridinium chloride in Pseudomonas stutzeri and changes in antibiotic susceptibility. J Hosp Infect. 1999;42(3):219-229. doi:10.1053/jhin.1999.0591
- Gilbert P, McBain AJ. Potential Impact of Increased Use of Biocides in Consumer Products on Prevalence of Antibiotic Resistance. Clin Microbiol Rev. 2003;16(2):189-208. doi:10.1128/CMR.16.2.189-208.2003
- Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Dose requirements for UVC disinfection of catheter biofilms. Biofouling. 2009;25(4):289-296. doi:10.1080/08927010802716623
- Li J, Hirota K, Yumoto H, Matsuo T, Miyake Y, Ichikawa T. Enhanced germicidal effects of pulsed UV-LED irradiation on biofilms. J Appl Microbiol. 2010;109(6):2183-2190. doi:10.1111/j.1365-2672.2010.04850.x
- Bae YM, Lee SY. Inhibitory effects of UV treatment and a combination of UV and dry heat against pathogens on stainless steel and polypropylene surfaces. J Food Sci. 2012;77(1):61-64. doi:10.1111/j.1750-3841.2011.02476.x
- Hadjok C, Mittal GS, Warriner K. Inactivation of human pathogens and spoilage bacteria on the surface and internalized within fresh produce by using a combination of ultraviolet light and hydrogen peroxide. J Appl Microbiol. 2008;104(4):1014-1024. doi:10.1111/j.1365-2672.2007.03624.x
- Flemming H-C. Microbial Biofouling: Unsolved Problems, Insufficient Approaches, and Possible Solutions. In: Flemming H-C, Wingender J, Szewzyk U, eds. Biofilm Highlights. Berlin Heidelberg: Springer – Verlag; 2011:81-109. doi:10.1007/978-3-642-19940-0_5
- Simões M, Simões LC, Machado I, Pereira MO, Vieira MJ. Control of Flow-Generated Biofilms with Surfactants. Food Bioprod Process. 2006;84(4):338-345. doi:10.1205/fbp06022
- Gora SL, Rauch KD, Ontiveros CC, Stoddart AK, Gagnon GA. Inactivation of biofilm-bound Pseudomonas aeruginosa bacteria using UVC light emitting diodes (UVC LEDs). Water Res. 2019;151. doi:10.1016/j.watres.2018.12.021
- US Enviromental Protection Agency Office of Pesticide Programs. Standard operating procedure for growing a Pseudomonas aeruginosa biofilm using the CDC biofilm reactor. Off Pestic Programs. 2013:1-14.
- Gagnon GA, Slawson RM. An efficient biofilm removal method for bacterial cells exposed to drinking water. J Microbiol Methods. 1999;34(3):203-214. doi:10.1016/S0167-7012(98)00089-X
- Rattanakul S, Oguma K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms. Water Res. 2018;130:31-37. doi:10.1016/j.watres.2017.11.047
- Bak J, Ladefoged SD, Tvede M, Begovic T, Gregersen A. Disinfection of Pseudomonas aeruginosa biofilm contaminated tube lumens with ultraviolet C light emitting diodes. Biofouling. 2010;26(1):31-38. doi:10.1080/08927010903191353