Tatiana Koutchma
Research Scientist, Agriculture and Agri-Food, Canada
Ultraviolet-C (UV-C) light is emerging in food and beverage processing as a nonthermal, nonchemical and nonionizing organic technology that offers a less expensive, energy-saving alternative solution to heat for treatment of liquid ingredients, juice products and other beverages. Compared to another nonthermal process, such as high hydrostatic pressure (HHP), UV-C has an advantage of continuous mode treatment and offers packaging flexibility. A large number of data reported has shown the effectiveness of UV-C light at 253.7 nm against foodborne pathogens, natural microbiota, moulds and yeasts. Also, due to its nonthermal nature, UV-C processing resulted in better product quality, higher nutritional content and fresh-like sensory attributes of products compared to thermal processing.
Despite these obvious benefits, existing regulatory approvals in the US, Canada and European Union, as well as successful experience of application of UV-C for municipal and wastewater treatment, commercialization of UV-C for opaque beverages has been slow. This article will discuss the knowledge gaps in product and UV-C process development that need to be addressed to accelerate technology commercialization. Also, the essential differences in water and beverage product characteristics and process requirements will be discussed and must be considered for successful UV-C process development and validation of opaque beverages. This will include major requirements for UV-C preservation of beverages, principles of establishment of equivalent preservation UV-C dose and quality effects, challenges of process validation and effective designs of UV-C systems.
Requirements for UV-C preservation of beverages
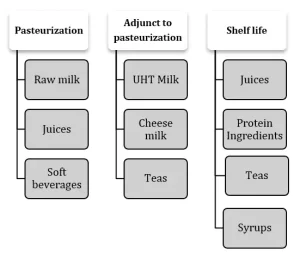
Developing a new UV-C process requires initially establishing a proper design and operational dose followed by a validation step to ensure that UV-C dose is reliably delivered to the product and evaluation of the microbial efficiency of a system. Unlike in water treatment, the UV-C dose for beverages has not been established by the regulators, and the process must be developed based on achievement of product-specific process requirements, or so-called intended technical effects. The intended technical effect for liquid foods and beverages can require addressing food safety issues, often through a pasteurization step, and achieving shelf life extension at refrigerated or ambient storage conditions. This can involve a 5 log target pathogen reduction in juice products to comply with HACCP (US FDA 2001), shelf life extension of fresh juices or adjunct treatment for pasteurized milk, teas or liquid sugars by eliminating heat-resistant spores or spoilage organisms, as shown in Figure 1.
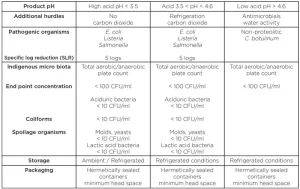
Since preservation and storage requirements are very much defi ned by the product pH, the examples for three pH categories of juices and soft beverages are shown in Table 1.
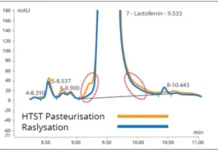
 In the case of using UV-C light for milk preservation, the process requirements will be defined by the specific operation in milk processing where UV-C can be applied, such as thermization, pasteurization, ESL (extended shelf life) treatment or sterilization step for chilled or ambient distribution. Also, the UV process should be optimized to achieve the best quality, nutritional and sensory attributes. After the UV-C operational dose is established, its effects on product enzymes and stability of the suspension should be taken into consideration for improved product quality during storage.
In the case of using UV-C light for milk preservation, the process requirements will be defined by the specific operation in milk processing where UV-C can be applied, such as thermization, pasteurization, ESL (extended shelf life) treatment or sterilization step for chilled or ambient distribution. Also, the UV process should be optimized to achieve the best quality, nutritional and sensory attributes. After the UV-C operational dose is established, its effects on product enzymes and stability of the suspension should be taken into consideration for improved product quality during storage.
Establishment of preservation reduction equivalent UV-C dose
A general approach for the establishment of the UV-C preservation process for beverages includes these steps:
1. Identification of the organisms of concern (pathogenic and spoilage) and their UV-C resistance
 The choice of the organism of concern in juice and other beverage products depends on product pH and will differ for high-acid, acid and low-acid beverages as shown in Table 1. Knowledge of the food mformulation and history of the food outbreaks and/or evidence of potential growth is essential when selecting the appropriate challenge pathogens. Multiple specific strains of the target pathogens should be included in the challenge study, and their UV-C resistance must be measured to select the most UV-C resistant strains. It is typical to challenge a food formulation with a “cocktail” or mixture of multiple strains in order to account for potential strain variation. The ideal organisms for challenge testing are those that have been previously isolated from similar formulations, and pathogens from known foodborne outbreaks should be included to ensure the formulation is robust enough to inhibit the growth of these organisms.
The choice of the organism of concern in juice and other beverage products depends on product pH and will differ for high-acid, acid and low-acid beverages as shown in Table 1. Knowledge of the food mformulation and history of the food outbreaks and/or evidence of potential growth is essential when selecting the appropriate challenge pathogens. Multiple specific strains of the target pathogens should be included in the challenge study, and their UV-C resistance must be measured to select the most UV-C resistant strains. It is typical to challenge a food formulation with a “cocktail” or mixture of multiple strains in order to account for potential strain variation. The ideal organisms for challenge testing are those that have been previously isolated from similar formulations, and pathogens from known foodborne outbreaks should be included to ensure the formulation is robust enough to inhibit the growth of these organisms.
It is not unusual to have a cocktail of five or more strains of each target pathogen in a challenge study. A benchtop collimated beam set-up can be used to measure UV-C resistance in the tested medium. However, the essential modifications to the standard water protocol must be made due to high UV absorbance of beverages and to achieve uniform UV-C dose delivery in the sample. Currently – in the absence of established research methods, standard approaches, bio safety level 2 and 3 labs, as well as resources to conduct microbial UV inactivation studies in opaque beverages – a large variance in UV-C resistance of foodborne pathogens can be observed among reported data. However, in the proper set-up and correctly designed biodosimetry experiments, a first-order linear model adequately describes the destruction of organisms for many beverages under the action of UV-C, or inactivation rate constants should be determined using adequate nonlinear models.
2. Identification and selection of the appropriate reduction target – safety and shelf life
As indicated above, for high-acid juices, the 5 log reduction of pathogenic Escherichia coli, Salmonella and Listeria has to be demonstrated to comply with US FDA HACCP rules. For low-acid products, the process must be capable of reducing 5 logs of nonproteolytic Clostridium botulinum. With respect to reduction requirements of spoilage and natural microbiota, the normal initial levels seen in beverage processing operations have to be evaluated and the recommended end point approach should be used to determine the margins of the process (Table 1).
The possibility of growth of selected organisms in target products also should be explored. Records of normal microbial counts in industrial food products are known for their irregular, fluctuating character that is determined primarily by variations in the initial load and numerous random factors, which tend to promote or inhibit the microbial growth. Since understanding of the complex and dynamic microbial ecosystems in food production environments is still relatively superficial, food preservation and safety assurance systems must remain crude and be guided by general principles that apply to both spoilage and pathogenic species.
3. Development of a conservative estimation of UV-C dose to deliver the targeted microbial reduction
Preservation reduction equivalent UV-C dose![]() has to be determined and applied to meet a required specific logarithmic microbial reduction (SLR) in numbers of target pathogenic organisms or end point concentration of spoilage organisms using eq. 1 and 2.
has to be determined and applied to meet a required specific logarithmic microbial reduction (SLR) in numbers of target pathogenic organisms or end point concentration of spoilage organisms using eq. 1 and 2.
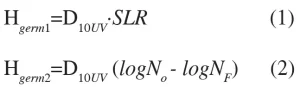
According to the equation (1),![]() is the amount of UV-C energy in mJ/cm-2 that must be delivered to pathogenic organisms in the product to achieve SLR.
is the amount of UV-C energy in mJ/cm-2 that must be delivered to pathogenic organisms in the product to achieve SLR. ![]() is a UV-C dose to reduce 90% of relevant pathogenic population with the highest UV-C resistance. The germicidal dose
is a UV-C dose to reduce 90% of relevant pathogenic population with the highest UV-C resistance. The germicidal dose![]() is the UV-C dose needed to achieve the end point concentration of spoilage organisms and is a function of the initial load
is the UV-C dose needed to achieve the end point concentration of spoilage organisms and is a function of the initial load![]() , the end point of the process
, the end point of the process![]() and the resistance of target spoilage bacteria under defined conditions (
and the resistance of target spoilage bacteria under defined conditions (![]() ). It was reported that typically the UV-C resistance of spoilage yeast and mold spores is much higher than pathogenic bacteria. Also, it is recommended that the highest value of
). It was reported that typically the UV-C resistance of spoilage yeast and mold spores is much higher than pathogenic bacteria. Also, it is recommended that the highest value of![]() or
or![]() be used as the UV-C preservation reduction equivalent dose.
be used as the UV-C preservation reduction equivalent dose.
Determination of the critical factors to control the delivery of the required process
The actual preservation UV-C dose can vary for different categories of juices, beverages or liquid ingredients and very much depends on product composition, absorption and scattering properties, rheological characteristics, initial microbial load and reduction requirements. Within a food product, several factors can influence the delivery of the UV-C dose, such as pH, soluble and suspended solids, and product composition. The presence of particles could provide a means of shielding microorganisms from UV light photons.
A few factors found to consistently affect the efficacy of UV inactivation in opaque beverages were product pH, absorbance and scattering or product UV-T, viscosity and temperature. Changes and variations in UV absorbance of food products should be considered, and a simple absorbance reading may prove inadequate to predict the microbial destruction within most beverage systems. Physical properties of fluids, such as viscosity and density, influence the effectiveness of momentum transfer and flow pattern in the UV chamber. Additionally, product viscosity impacts the hydrodynamic behavior of the liquid in UV systems and, consequently, the delivery of UV photons.
Characterization of physical, optical and biochemical properties of beverages is highly desirable for proper process establishment. Sufficient UV absorbance data of beverages should be properly collected, characterized and reported. The absorbance measurement techniques should account for the presence of the suspended particles and soluble solids and their effect on the estimation of the UV-C dose. In addition, the effect of some essential compounds, such as vitamin C, on the absorption effects of the product needs to be considered during UV treatment. In determination of the UV absorbance design criteria, the full range of UV absorbance at the UV implementation point where the system will be installed should be determined. This enables selection of an appropriate UV-T range to ensure compliance at all times.
Validation of UV-C process for beverages
The primary objective of validation is to demonstrate UV-C process consistency to deliver the required performance over time across R&D scales and manufacturing environments with various raw materials and operating ranges. Similar to established practices in UV water treatment, dose delivery by the system can be assessed using biodosimetry.
With biodosimetry, inactivation of challenge microorganisms is measured and related to a 90% reduction dose or reduction equivalent dose using the known UV-C dose-response. Unless working in a controlled environment (e.g., a Biosafety level 2/3 pilot scale containment facility), pathogens should not be used in an open processing environment with a potential risk for contamination of equipment and facilities. In these situations, nonpathogenic “surrogate” organisms or challenge microorganisms are selected for use in lieu of the pertinent pathogen.
According to the US FDA (2000) the surrogate microorganism is defined as “nonpathogenic species and strain responding to a particular treatment in a manner equivalent to a pathogenic species and strain.” Obviously, such challenge strains must have resistance traits that have been predetermined in controlled studies to match as closely as possible those of the pertinent pathogen. If a surrogate strain is to be used in a microbiological validation study, preliminary work should be done to well characterize the strain before use in the validation study. In the current situation, the number of validation studies of UV-C processing of beverages is very limited, and no guidelines or protocols have been developed so far to assist researchers and food processors.
Also, it is important to recognize that each preservation process is unique and suggests that validation studies will be process- and product-specific. It is the responsibility of the food processors and process authority to demonstrate the ability of their process to achieve the specific (required) log reduction of the pertinent microorganisms in the product. However, the developing validation guidelines should lead to significant commercial benefits for the manufacturers and UV-C technology providers.
UV-C impact on quality, composition and sensory
![]()
 Despite the promising status and existing regulatory approvals, the commercialization of UV-C technology requires acceleration through research emphasis on the physical characteristics, quality, nutrients, enzymatic and microbial stability and the composition changes in UV-C treated products. Generally, the UV-C treatment is not considered to pose any new nutritional safety concerns when used at the doses required to achieve 5 log reduction of pathogenic organisms in high-acid juices and beverages. Also, most studies concluded that, when compared to thermal processing, UV treatment is able to better preserve the quality and nutritional attributes.
Despite the promising status and existing regulatory approvals, the commercialization of UV-C technology requires acceleration through research emphasis on the physical characteristics, quality, nutrients, enzymatic and microbial stability and the composition changes in UV-C treated products. Generally, the UV-C treatment is not considered to pose any new nutritional safety concerns when used at the doses required to achieve 5 log reduction of pathogenic organisms in high-acid juices and beverages. Also, most studies concluded that, when compared to thermal processing, UV treatment is able to better preserve the quality and nutritional attributes.
However, it is known that UV-C light can potentially adversely affect food products by generating free radicals by a wide variety of organic photochemical reactions. These adverse effects are very much determined by the UV-C dose and, in some cases, the level of UV-C irradiance. Depending on the UV processing dose and specific nutrient(s), UV light may have a positive, neutral or negative effect on nutrient and enzyme retention. The key knowledge for turning the detrimental effects of UV-C light into beneficial ones could lie in light-control of biological and chemical systems such as enzymes and other compounds. In order to determine the most beneficial use of UV light, it is necessary to test each product for its UV spectral response. Special attention should be given to the beneficial effects of UV-C light, such as increase in enzyme activity, enhancement of some antioxidants and vitamin content, and color improvement after treatment and during storage. In addition, knowledge of the degradation kinetics of vitamins, enzymes and bioactive compounds by UV-C light will allow optimization of microbial inactivation of pathogenic and spoilage organisms while minimizing losses of these health-related compounds. Also, sensory studies should be conducted in commercial scale units to improve consumer acceptance. These data are critical for regulatory reviews of novel foods treated using UV-C technology, new product and process development, and product storage stability.
UV-C system design
Due to low UV-T and viscosity challenges, novel UV-C systems for opaque beverages have to be specifically developed and differ from designs normally employed for water treatment. Thin film laminar, annular turbulent, static and dynamic mixing regimes are used to effectively treat juice suspensions and beverages. Currently, only a few designs of continuous flow UV apparatus are available for processing various types of these products. Some of these UV-C systems are under development or testing, and only a few are commercially available. Limited information has been reported on the application of commercial scale UV-C systems, including CiderSure (thin film laminar flow; FPE, NY, USA), Salcor module (Dean flow in coiled tube, turbulent; CA, USA), SurePure (thin film, turbulent; SurePure Inc., Zug, Switzerland) and AseptoRay (static mixer, turbulent, MGT, Israel). Food processors that are interested in using UV-C technology must select the most appropriate system for the application and establish processing conditions to achieve safety and shelf life objectives. At the same time, processing conditions have to provide the required level of quality while retaining nutritive constituents, as well as product sensory and structure attributes.
Next steps
According to the reported effects on pathogenic and natural microbiota, UV-C treatment of opaque beverages can potentially provide safety levels and shelf life of products during refrigerated storage similar to those for products treated with HHP with no effects on the beverages’ essential quality, nutritional and sensory attributes. The lower initial investment, operating costs, packaging flexibility and size requirements associated with industrial UV-C treatment show the feasibility of this technology as the primary non-thermal processing alternative for the premium beverages category. Due to the limited data of UV-C resistance of foodborne pathogens and absence of common established practices to measure and evaluate UV-C inactivation kinetics in opaque beverage products, the challenge remains to properly establish UV-C preservation process and obtain regulatory approvals.
Because the UV-C dose has not been established by regulators, individual UV-C processes must be developed and validated based on achieving product-specific requirements. Therefore, the main challenge for food processors interested in using UV technology is to select the most appropriate UV-C system for the application and establish processing conditions to achieve appropriate safety, shelf life and quality objectives. The development of validation and verification practices – such as phosphatase test in milk pasteurization, optimization of UV equipment and further regulatory approvals – can certainly bring the UV-C technology for beverages closer to commercial implementation.
References
- Anonymous (2000). Kinetics of microbial inactivation for alternative food processing technologies. Institute of Food Technologists. J. of Food Science Supplement http://vm.cfsan.fda.gov/~comm/ift-pref.html.
- US FDA. 2001. Hazard analysis and critical control point (HACCP): Procedures for the safe and sanitary processing and importing of juice. Final rule. Federal Register, 66, No.13. US Food and Drug Administration, Washington, DC.
Contact: Tatiana.koutchma@canada.ca