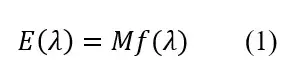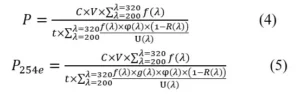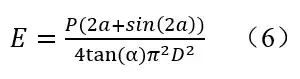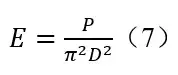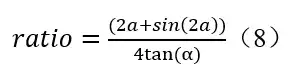By Lianfeng Zhang, Laboratory of Ecology and Environmental Protection, Research Institute of Tsinghua University
When measuring the output of UV LEDs in a photoelectric radiometer, the probe matched to the specific UV LED wavelength is required. The measurement result of the radiometer is the irradiance of the irradiated point. An understanding of the radiation model (mathematics) always is required in order to convert the measured irradiance to the UV output of the UV LED. However, each type of UV LED has its specific emission characterized by wavelength, full width at half maximum (FWHM) and radiation model.
The KI/KIO3 actinometer can provide a solution to such problems. If the measurement setup is set up properly, the liquid of the KI/KIO3 actinometer has the potential to capture all the photons emitted by the UV LED. This measurement method has been established and will be published. 1, 2
Due to manufacturing characteristics, the tubes of Kr-Cl excimer lamps generally are short and thick (large diameter). When measuring its UV output in the IUVA protocol 3, the probe must be set far away. In this case, the radiation on the probe is too weak for accurate measurements. If the irradiation time is prolonged, the KI/KIO3 actinometer can solve this problem. 4 This article presents the core of the approaches.
The solution of chemical actinometer can be turned to form a vortex. If the UV LED is set inside the vortex of the solution of KI/KIO3 chemical actinometer, all photons emitted from the UV LED will be absorbed, regardless of radiation models. All photons would be absorbed in the actinometer solution for wavelengths < 290 nm. 5 When measuring Kr-Cl excimer lamps, the radiation model has been set in the IUVA protocol. 3
The KI/KIO3 chemical actinometer is the solution of KI, KIO3 and buffer (Na2B4O7). After it receives ultraviolet rays with a wavelength less than 330 nm, the iodide ions in the solution will undergo a photochemical reaction to generate triiodide complex (I3-). Because triiodide complex has a large absorption at 352 nm, by measuring the absorbance at 352 nm, the concentration of complex can be determined according to Lambert-Beer law, thereby obtaining the number of photons entering the liquid.
Radiation spectrum
The spectrum of a UV LED or a Kr-Cl excimer lamp can be measured by a spectrometer. It can be expressed as
f(?) is a relative value curve with a maximum (peak) value of 1 on the radiation spectrogram; M is a coefficient that converts relative values f(?) into absolute values (W).
Quantum yield
After the absorbances of the chemical actinometer at 352 nm before and after ultraviolet irradiation are measured, the concentration triiodide complex can be found
?A352 is the difference in the absorbance of the chemical actinometer at 352 nm before and after ultraviolet irradiation; e352 is the absorbance coefficient of the chemical actinometer at 352 nm; C is the concentration of triiodide complex (M). The quantum yield (f(?)) can transfer the concentration of triiodide complex into the energy received by the chemical actinometer. 6
Reflection coefficient
On the surface of the vortex, the reflection coefficient of 0.025. 2 In the measurement of the Kr-Cl excimer lamp, the beams are approximately parallel. In this case, the reflection coefficient can be considered as 0.025. 7
Energy of photons
The energy possessed by one mole of photons is related to the wavelength of photons.
NA is Avogadro’s constant (6.02214076×1023/mol-1), h is the Planck constant (6.62607015×10-34 J•s), c is the speed of light (2.99792458×108 m/s), and ? is the wavelength (nm).
Germicidal effectiveness factor
Different wavelengths of UV photons have different disinfection effects, which can be corrected by the disinfection effect coefficient (g(?)). 8,9 Since the disinfection effect is relative, the disinfection effect coefficient of 254 nm photons can be set as 1.0, and the disinfection effect coefficients of other wavelengths are relative values, relative to the 254 nm. The measured total energy of UV photons emitted by the UV LED then can be converted to the energy of 254 nm photons with the same disinfection power. That is, multiply the radiation energy by the disinfection effect coefficient.
The overall formulas
Combining all coefficients, the overall formulas for the power falling onto the surface of the chemical actinometer can be determined. 1,2
P is the UV power that falls onto the surface of the chemical actinometer (W); P254e is the power of the corresponding 254nm photon when the same disinfection effect is achieved (W); V is the volume of the chemical actinometer (mL); t is the irradiation time (s). The spectrum and disinfection coefficient were introduced into the formula, so the measured values can be used to compare the disinfection effects among various UV LEDs.
Keitz equation and point light source
When measuring the UV output of Kr-Cl excimer lamps according to the IUVA protocol 3, the chemical actinometer has to be set far away. In that case, the measurement distance becomes an issue. Keitz equation of the IUVA protocol can be mathematically transformed as
E is irradiance (mW/cm2); D is distance from lamp center to the UV sensor (cm); P is the lamp output power; a is the half angle (radians) subtended by the lamp at the sensor position. 3 The theoretical base of the Keitz equation is the assumption that the emission observes Diffuse radiation model. In such Diffuse model, for a point light source 10
According to formulas (6) and (7), the calculated irradiance ratio of the line light source (Keitz equation) to the point light source is
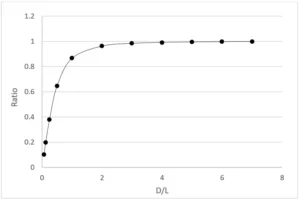
Figure 1 shows the values at different D/L. It can be seen from Figure 1 that when D/L>=4, the calculation results of the line light source (Keitz equation) and the point light source are the same; that is, when the irradiation distance increases, there is no need to consider changing the calculation formula from the line light source to the point light source. In IUVA protocol, the radiation distance was set below four times the lamp length.
The main consideration is that when it is too far, the ultraviolet radiation is weak, which will increase the measurement error of the radiometer. In the case of chemical actinometer, the irradiation time can be increased and then error will not be increased due to the weak irradiance.
References
- Zhang L, Zhou Y, Chang B et al. A technique for direct measurement of UV output of UV LED with KI / KIO3 chemical actinometer. Acta metrological sinica, 2023a, in press.
- Zhang L, Zhou Y, Chang B, et al. Determination of the reflection loss at gas-liquid interface in the measurement of UV LED by KI/KIO3 chemical actinometer. Acta metrological sinica, 2023b, in press.
- Lawal O, Dussert B, Howarth C, et al. Method for the Measurement of the Output of Monochromatic (254 nm) Low-Pressure UV Lamps. IUVA News, 2017. 19 (1): 9–16.
- Zhang L, Liao H, Yao S, et al. Measurement of far UV Output from Kr-Cl excimer lamp by KI/ KIO3 chemical actinometer. China light & lighting, 2023c, in press.
- Bolton J R. Personal communication between him and the author, the author has got his permission to cite publicly, 2022.
- Goldstein S, Rabani J. The ferrioxalate and iodide–iodate actinometers in the UV region (J). Journal of Photochemistry and Photobiology A: Chemistry, 2008, 193(1): 50-55.
- Bolton J R, Linden K G. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. Journal of Environmental Engineering, 2003. 129 (3): 209–215.
- Bolton J R. Terms and definitions in ultraviolet disinfection. Proceedings of the Water Environment Federation, 2000, 2000(2): 25-40.
- CIE (International commission on illumination). CIE 155:2003 Ultraviolet air disinfection. 2003.
- Zhang L, Anderson W A. A finite model for the prediction of the UV radiation field around a linear lamp. Chemical Engineering Science, 2010, 65: 1513–1521.
Lianfeng Zhang is the director at the Ecology and Environmental Protection Laboratory in the Research Institute of Tsinghua University in Shenzhen. Zhang completed bachelor’s and master’s degrees in China before pursuing a doctoral degree in Japan and conducting postdoctoral research in Canada. In 1993, Zhang moved to Japan and spent eight years focusing primarily on research related to water treatment. The doctoral thesis revolved around the subject of photocatalysts, which sparked dedication to the exploration and advancement of ultraviolet (UV) and advanced oxidation technologies. Following the time in Japan, Zhang joined the University of Waterloo in Canada as a postdoctoral researcher, specializing in advanced oxidation technologies and UV measurement; accepted a position as a research and development engineer at a prominent manufacturer of UV products in the United States; joined a water treatment company in China; and then transitioned to the Research Institute of Tsinghua University. In 2020, Zhang developed an underwater UV lamp measurement technology independently, which motivated the establishment of the IUVA Task Force “UV Measurement Underwater.” For more information, email tyou6@hotmail.com or call +86-18823700816.