Harold Wright, chief technologist- UV disinfection, Carollo Engineers
Traci Brooks, lead technologist, Carollo Engineers
Mark Heath, principal technologist, Carollo Engineers
Jeff Adams, environmental engineer, USEPA, ORD, NRMRL, WSWRD, WQMB
Bryan Townsend, UV technology leader, Black & Veatch Corporation
The US Environmental Protection Agency recently published the document titled “Innovative Approaches for Validation of Ultraviolet Disinfection Reactors for Drinking Water Systems.” This document is the result of a four-year project, funded by EPA, to develop and document new approaches for UV dose monitoring and validation that incorporates advances developed since the publication of the 2006 UV Disinfection Guidance Manual (UVDGM). Prior to publication, the document completed a two-year stakeholder review that was led by IUVA and included regulators, academia, consultants and UV system manufacturers. The final document was approved for publication based on the results of a stakeholder survey and an internal review by EPA.
This article provides background on the drivers for the document and provides a summary of the key benefits for the UV industry.
Background
UV technologies are used to provide disinfection and advanced oxidation for drinking water, wastewater, and potable and non-potable reuse applications. With many of those applications, utilities and regulators select UV reactors that use UV dose monitoring algorithms that have been developed and demonstrated through UV validation testing, conducted in accordance with established protocols that include the EPA’s UV Disinfection Guidance Manual (UVDGM) (USEPA, 2006) and the German and Austrian UV Rules (DVGW, 2006; ÖNORM, 2001; ÖNORM, 2003) for drinking water applications and the NWRI UV Guidelines (NWRI and WRF, 2012) for non-potable reuse applications.
The UVDGM describes two approaches for UV dose monitoring and validation: The UV intensity setpoint approach and the calculated dose approach. The UV intensity setpoint approach is based on the monitoring approach specified by the German and Austrian rules. With this approach, the UV reactor delivers a required UV dose when the UV intensity measured by the UV sensor is greater than or equal to a setpoint value that is defined as a function of the flow rate through the UV reactor. With the calculated dose approach, validation test data is used to develop an equation that predicts the reduction equivalent dose (RED) delivered by the reactor as a function of the flow through the reactor, the UV transmittance (UVT) of the water being treated and the UV intensity measured by UV sensors.
When the UVDGM was being prepared, there was limited experience developing equations for the calculated dose approach using validation data. The UVDGM stated that the following empirical equation can be used to fit validation data:
![]()
where A254 is the UV absorption coefficient at 254 nm, S is the measured UV sensor value, S0 is UV sensor value at 100% lamp power with new lamps and clean sleeves, Q is flow rate, B is the number of operating banks of lamps within the UV reactor, and a through e are model coefficients. The UVDGM defined the validated UV dose as:
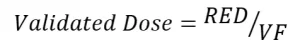
where VF is the validation factor. To achieve a target pathogen inactivation, the validated dose needed to be greater than the UV dose required by the regulations.
The validation factor accounts for uncertainties and biases that occur when experimental testing is used to define the validated dose delivered by the reactor. The biases include the RED bias, which applies to all UV systems regardless of lamp type, and the polychromatic bias, which only applies to UV systems using polychromatic lamps (e.g., medium pressure [MP] UV lamps).
The RED bias occurs when the UV dose-response at 254 nm of the validation test microbe differs from that of the pathogen. If the validation test microbe is more resistant to UV light than the pathogen, the RED measured during validation will be greater in value than that delivered to the pathogen. To address this issue, the UVDGM provides tables of RED bias correction factors that can be incorporated within the validation factor. However, those values are based on a UV reactor with a relatively wide UV dose distribution and are conservative for many commercial reactors. To minimize RED bias, the UVDGM recommends validation with multiple microbes whose UV dose-response brackets that of the target pathogen. With this approach, the validation data can be interpolated to eliminate the need to apply the RED bias. However, the interpolation approach recommended by the UVDGM at the time of publication was neither well defined nor practical.
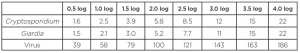
While there are several sources of polychromatic bias documented within the UVDGM, the most significant bias occurs when the wavelength response or action spectrum of the validation test microbe differs from that of the target pathogen. When the UVDGM was published, there was limited data on the wavelength response of validation test microbes and target pathogens. Analysis provided in the UVDGM indicates the bias is small when validation using MS2 phage was used to show inactivation of Cryptosporidium. However, research since has shown there are significant differences in the action spectra of validation test microbes and Cryptosporidium, Giardia and adenovirus.
The final report for Water Research Foundation Project 4376 (Linden et al., 2015) provides tables of action spectra correction factors (ASCFs) that can be incorporated within the validation factor to account for these issues. However, like the RED bias factors in the UVDGM, they were conservatively defined based on a worst-case UV reactor. Furthermore, because UV sensors do not provide proper monitoring at low wavelengths below 240 nm, the ASCF values were defined assuming UV provides no benefits at those wavelengths, adding an extra level of conservatism.
New approaches for monitoring and validation
The “Innovative Approaches” document describes four approaches for UV dose monitoring that have been developed since the UVDGM was published in 2006. With the first approach, applicable to UV systems using low-pressure (LP) and MP lamps, log inactivation is modeled as a function of the UV absorbance (UV-A) of the water, calculated from the UVT, and a combined variable, (S/S0)/(Q DL), where DL is the UV dose per log inactivation of the microbe. An example of this equation is:
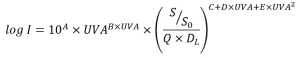
where A though E are constants obtained by fitting the equation to the validation data. The RED is predicted using:
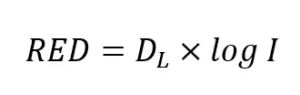
This approach for analyzing validation data was developed in 2007 (Bircher and Wright, 2007) and since has been applied to more than 35 closed-vessel UV reactor product lines.
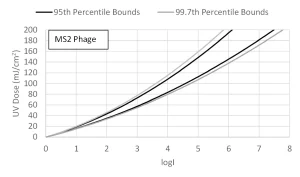
In concept, equations using the combined variable can be calibrated using validation conducted with one challenge microorganism, such as MS2 phage, and then used to directly predict the log inactivation of the target pathogen by setting the value of DL to that of the target pathogen.
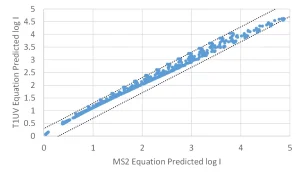
In practice, the equation should be calibrated using a validation dataset collected using two or more challenge microorganisms with different UV dose-responses, such as MS2 and T1UV phage. The ability of the equation to predict the log inactivation of target pathogens using the DL of those pathogens can be tested by showing that the equation calibrated using MS2 phage predicts the log inactivation of T1UV phage and vice versa (Figure 1).
Using the equation to directly predict the log inactivation of the target pathogen, such as Cryptosporidium or adenovirus, means that the RED bias can be set to 1.0, which simplifies application of the validation factor and facilitates the most cost-effective selection and application of UV technologies. The approach also can be used to predict the inactivation of other target pathogens that may be relevant in other industries and jurisdictions, such as rotavirus.
With the second approach, the log inactivation at a given UVT is expressed as a function of a combined variable, S/(Q DL).
If the UV sensor is optimally located within the UV reactor, the relations between log inactivation and the combined variable, S/Q/DL, at various UVTs tend to overlap, and a single relation can be used to define a UV monitoring algorithm that does not require an online UVT monitor (Wright et al., 2009). If the relations do not perfectly overlap, the single relation can be defined conservatively based on the minimum log inactivation at a given value of the combined variable. An example of this type of algorithm is:
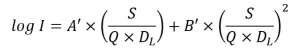
where A’ and B’ are constants obtained by fitting the equation to the minimum log inactivation values. Further simplification is obtained by setting the value of DL to a target pathogen, thereby setting the RED bias factor to 1.0, and incorporating the validation factor, which results in an algorithm specific to a target pathogen (Figure 2), such as:
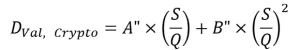
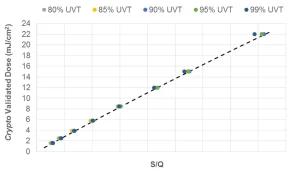
The last two approaches are applicable with MP UV systems and use low and high wavelength UV sensors to monitor the contribution to UV dose delivery by wavelengths below and above 240 nm, respectively.
These approaches also use low and high wavelength ASCFs that are calculated using the action spectra of the validation microbe and target pathogen as reported in WRF Project 4376 or calculated using UV dose-response data measured using a collimated beam apparatus equipped with a MP lamp. If the low wavelength UV sensor is optimally located, a low wavelength UVT monitor is not required.
While these approaches are more complex than those that only use a high wavelength UV sensor, they do provide disinfection credit for low wavelengths, which can be significant with pathogens like adenovirus.
Benefits for regulators and utilities
The monitoring approaches described in the “Innovative Approaches” document will improve the application of UV disinfection systems for the inactivation of Cryptosporidium, Giardia and viruses. The document provides a reference for new and enhanced validation methods developed since the publication of the UVDGM.
These methods promote standardization and enhanced accuracy for UV dose monitoring, and in many cases, will simplify the application of UV disinfection.
Research needs
The document includes a list of research needs ranging from the application of computational fluid dynamics (CFD) to complement validation testing to UV monitoring and validation for UV systems using LEDs. Areas of research not listed include how to account for the increase in UVT through the UV reactor on monitoring that occurs with UV advanced oxidation process (AOP) applications (Wright et al., 2018) and limitations of the combined variable approach with open channel UV systems (Wright et al., 2020).
Disclaimer
The U.S. Environmental Protection Agency, through its Office of Research and Development, funded and managed, or partially funded and collaborated in, the research described herein. It has been subjected to the Agency’s peer and administrative review and has been approved for publication. Any opinions expressed are those of the author(s) and do not necessarily reflect the views of the Agency, therefore, no official endorsement should be inferred. Any mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Contact: Harold Wright, [email protected]; Traci Brooks, [email protected]; Mark Heath, [email protected]; Jeff Adams, [email protected]; Bryan Townsend, [email protected]
References
- Bircher, K. and H. Wright. 2007. Who needs RED? An empirical method for validating log-inactivation. Presented at the 2007 Water Quality Technology Conference, Charlotte, NC, November 4 to 8, 2007.
- Brooks, T., Wright, H., Heath, M., Larner, S. and P. Ropic 2019. UV Dose Monitoring Simplified for Small Systems. IUVA World Congress, February 10-13, 2018, Sydney, Australia.
- DVGW. 2006. UV Disinfection Devices for Drinking Water Supply––Requirements and Testing. DVGW W294-1, -2, and -3. German Gas and Water Management Union (DVGW), Bonn, Germany.
- Linden, K.G., H. Wright, J. Collins, C. Cotton, and S.E. Beck. 2015. Guidance for Implementing Action Spectra Correction with Medium Pressure UV Disinfection. Water Research Foundation, Denver, CO.
- NWRI and WRF. 2012. Ultraviolet Disinfection Guidelines for Drinking Water and Water Reuse. Third Edition. National Water Research Institute, Fountain Valley, CA, in collaboration with American Water Works Association Research Foundation.
- ÖNORM. 2001. Plants for the disinfection of water using ultraviolet radiation—Requirements and testing—Part 1: Low pressure mercury lamp plants. ÖNORM M 5873-1. Osterreichisches Normungsinstitut, Vienna, Austria.
- ÖNORM. 2003. Plants for the disinfection of water using ultraviolet radiation—Requirements and testing—Part 2: Medium pressure mercury lamp plants. ÖNORM M 5873-2. Osterreichisches Normungsinstitut, Vienna, Austria.
- USEPA. 2006. Ultraviolet Disinfection Guidance Manual for The Final Long Term 2 Enhanced Surface Water Treatment Rule. EPA 815-R-06-007. U.S. Environmental Protection Agency, Office of Groundwater and Drinking Water, Washington, DC.
- Wright, H., Salveson, A. and E. Wicklein. 2018. Predicting Non-Steady State UV AOP with Potable Reuse Applications. 2018 WateReuse California Annual Conference, March 25-28, 2018.
- Wright, H., Brooks, T., Heath, M., Wicklein, E., Sotirakos, B. and A. Salveson. 2020. UV Disinfection Knowledge Base for Reuse Applications. Water Research Foundation, Denver, CO.