Liz Stevens, writer, UV Solutions
Staphylococcus aureus is one of many bacteria that can cause infection, and it is widely found in surgical settings and in patients with chronic non-healing wounds. Antibiotics have been effective against this bacterium in the past, but with the dramatic rise of antimicrobial resistant strains and especially the rise of Methicillin-resistant Staphylococcus aureus (MRSA), alternate methods for preventing and eradicating infection by the bacterium are necessary. MRSA is one of the first “superbugs” to have developed resistance to antibiotics because of overuse and inappropriate application of antibiotics for humans and for livestock. Disease and death in humans due to antimicrobial resistant pathogens is on the rise worldwide, regarded by some medical experts as a silent pandemic in the making.

Two presentations at the 2023 IUVA World Conference addressed the use of UV-C as a novel disinfection method against MRSA in surgical sites and for chronic wound management. “Application of 233 nm Far\UV-C wavelength for skin disinfection: Safety considerations” was presented by Loris Busch, Charité – Universitätsmedizin Berlin. “Innovative UV-C Phototherapy Platform for the Decontamination of Chronic Non-Healing Wounds” was presented by Mark Gerber, M.D., Spectrum Medical Technologies. UV Solutions talked with Busch and Gerber for their insight into the threat from MRSA and a high-level look at their research and developments.
In the Operating Room
In surgical settings, MRSA now is considered a prevalent, major threat. Loris Busch, a Ph.D. student in the field of skin physiology and pharmaceutical technology in the Center of Experimental and Applied Cutaneous Physiology, explained that Methicillin-resistant Staphylococcus aureus became strongly prevalent over the last few years, leading to high resistance rates in the Americas and Europe. “Since MRSA is a serious problem in nosocomial settings (originating in a hospital), the risk of a transfer to surgical sites exists,” Busch said. “Thus, MRSA often can be isolated from surgical site infections (SSI) occurring postoperatively. SSIs constitute a serious healthcare burden that also is associated with a heavy financial burden.” Dr. Gerber characterized the threat from MRSA in numerical terms. “According to the Center for Disease Control’s (CDC) Annual Antibiotic Resistant Threat Report from 2019 1 – the most current data available – there were 323,000 MRSA infections in the US, and this contributed to over 10,000 deaths,” Gerber said. “In fact, antibiotic-resistant infections in general have increased to the point that the CDC has started putting out an annual antibiotic resistance threat report. The rate of antibiotic resistant acquisition by pathogens is outpacing the rate at which we can develop new antibiotics, and it is just going to accelerate. It is a significant, significant problem.”
The usual methods for disinfecting a patient’s skin prior to surgery have proven to be inadequate for more than one reason. Topical iodine-containing antiseptics are just that – topical. They only are applied to the surface of skin and cannot disinfect the other areas where pathogens persist. “In 90% of all cases,” Busch explained, “SSIs are caused by endogenous pathogens (from within the affected person). Coming from pathogen reservoirs like the hair follicles, which cannot be reached by antiseptic solutions, they recolonize the skin during longer surgical interventions and thus may be transferred into the surgical wound intraoperatively.” Busch pointed out that one advantage of using a Far UV-C LED device for disinfection in the operating room is that, during longer surgeries, it could be used before and during surgery to keep the skin and surgical site clean. Gerber added that iodine-containing disinfectants are ill-advised for use with patients with thyroid disease or kidney disease. “In addition,” said Gerber, “iodine has been reported as harmful to fibroblasts, the human cells that initiate wound healing. Inhibiting those cells retards the healing process.”
Another common disinfectant, chlorhexidine, has its own drawbacks in addition to facilitating the very antimicrobial resistance at the heart of the MRSA matter. “Chlorhexidine can be effective against a lot of these pathogens,” Gerber explained, “however, it also has been shown to delay wound healing. And recent literature shows that MRSA as well as some other antibiotic resistant pathogens have developed resistance to chlorhexidine.” Gerber and Busch cited some surprising abilities shown by pathogens in reaction to chlorhexidine treatment. “Some skin antiseptics, like chlorhexidine,” said Busch, “harbor the risk of kicking off a pathogen’s cross-resistance to antibiotics.” Gerber noted that bacteria have another way of reacting to antibiotics that are not able to kill them. “If an administered antibiotic is not effective in deactivating a pathogen,” he said, “the pathogen can develop resistance to it. And then it can pass this resistance on to other bacteria through a process called horizontal gene transfer.”
The most stringent disinfection methods may fail to address the possibility of infection in operating rooms that stems from airborne pathogens, which could settle onto the surgical site. “The source here,” said Gerber, “is multifocal, including operating room personnel and the ‘stirring up’ of pathogens due to airflow within the OR.” 2, 3
Chronic Wound Infections
MRSA in patients with chronic wound infections also is a serious threat, conservatively estimated to afflict eight million people annually worldwide. 4 Gerber characterized chronic wound infections as those persisting for greater than 90 days, with osteomyelitis, amputations, sepsis and death among the outcomes if the infections are not eradicated. “MRSA wound infections are rapidly increasing,” said Busch, “which is why adequate topical treatment is highly demanded. Chronic wounds constitute a major economic and health burden.”
As with surgical site skin disinfection, the usual disinfection treatments for chronic wound infections now are inadequate. “The problem with chronic wounds,” said Gerber, “is that, in addition to the drawbacks of iodine-based solutions and chlorhexidine, a lot of patients with chronic wounds have vascular compromise. For these patients, antibiotics are contraindicated because antibiotics cannot get to the site of the infection in adequate concentrations due to the patients’ decreased blood flow.” Chronic wound infections can develop biofilm, a material secreted by the bacteria to protect themselves – an envelope which insulates the pathogens from topical treatments. Chronic wound infections also are often polymicrobial – a combination of different bacterial and fungal infections – leading healthcare providers to treat the infections with a cocktail of many antibiotics to address multiple pathogens. Doing this, however, douses pathogens with ineffective as well as effective antibiotics, allowing pathogens to develop further resistance to antibiotics. Horizontal gene transfer is an issue here, too. “Microbiostatic antiseptics like chlorhexidine gluconate, quaternary ammonium compounds or silver ions,” explained Busch, “are known to exhibit transferable resistances leading to cross-resistances with antibiotics.”
UV-C Disinfection
To combat MRSA in surgical settings and in critically colonized or chronic wounds, Busch and Gerber have each tested UV-C disinfection. At the 2023 IUVA World Conference, Busch presented results of his research in “Application of 233 nm Far UV-C wavelength for skin disinfection: Safety considerations,” co-authored by Johannes Schleusener, Silke B. Lohan, Marius Kröger, Daniela F. Zamudio Díaz, Neysha Lobo-Ploch, Claudia Sicher, Nevin Opitz, Paula Zwicker, Cornelia M. Keck and Martina C. Meinke.
Describing his and his colleagues’ work, Busch stated that “a very promising method for pre-surgical decolonization of the skin, as well as for the treatment of critically colonized or chronic wounds, is the use of 233 nm Far UV-C radiation by applying LED systems. At an application dose from 40 to 60 mJ/cm2, this radiation already shows complete eradication of Methicillin-resistant Staphylococcus aureus (MRSA) on agar plates and germ carriers.” Busch makes a strong distinction between UV-C at 233 nm and at 254 nm. “Unlike UV-C radiation of 254 nm, which is known for the disinfection of water and surfaces, 233 nm is strongly absorbed in the uppermost, non-nucleated layer of the skin, the stratum corneum.”
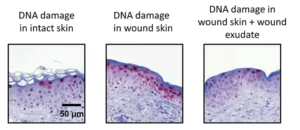
Busch’s group conducted a risk assessment of the effects of 233 nm radiation on skin, evaluating DNA damage in skin and the formation of free radicals, and published the results in Journal of Photochemistry and Photobiology B: Biology. 5 “233 nm Far UV-C showed only superficial DNA lesions in intact skin,” said Busch, “compared to UV-B [280-315 nm] and 254 nm radiation.” Radical formation was lower than for VIS-NIR irradiation, which is equivalent to a 20-minute stay in the noon sun under a cloudless sky. “Additionally,” Busch said, “no increase in the abundance and depth of DNA damage in wound skin covered by artificial wound exudate could be observed as compared to intact skin. In various skin types, the differences in DNA damage were much smaller than the differences after UV-B irradiation.” From these results, Busch’s group concluded that the application of UV-C at 233 nm on human skin at antimicrobial doses from 40 to 60 mJ/cm2 can be considered safe in various settings. A review article by Maximilian Görlitz et al summarizes the current knowledge on Far UV-C safety regarding skin and eye exposure. 6
“The UV-C lamp used for our experiments,” Busch explained, “is a novel developed LED system emitting at 233 nm. Currently, the system is not commercially available. It was developed by the FBH Institute in Berlin and the Technical University in Berlin.” 7 Busch’s group published evidence that in vivo application on humans is safe and effective, 8 and has published a paper on the safety aspects of using UV-C for disinfection of mucosa. 9 More testing is ongoing and partnerships with industrial suppliers with medical device regulation are being sought.
Delivering UV-C Disinfection
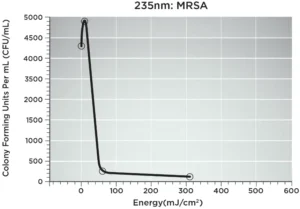
Dr. Gerber and co-author Leo Schowalter’s presentation, “Innovative UV-C Phototherapy Platform for the Decontamination of Chronic Non-Healing Wounds,” offered details on Spectrum Medical Technologies’ UV-C phototherapy platform – Surgical Site Light™ (SSL). This 235 nm UV-C LED device will deliver phototherapy as an alternative to traditional antibiotics in surgical settings and for chronic wound infections. Gerber tested the SSL against MRSA in swine and achieved a 94% decrease in colony forming units (CFUs) in 60 seconds (60mJ/cm2). Following an additional 250 seconds of exposure (total 310mJ/cm2), a >97% decrease in CFUs was observed. 235 nm UV-C exposure proved to be less detrimental to host tissue as compared with longer wavelength UV-C LEDs.
The SSL is a compact device designed for maximum disinfection, ease of use, portability and flexibility of LED architecture. “When I set out to design this device,” Gerber explained, “I kept in mind the possible clinical applications of UV-C LEDs – what is available now and what will be available in the future. The current LED architecture, produced by Crystal IS, emits light in the 235 nm wavelength. But if, in the future, a more effective wavelength is identified, the SSL can be produced with a different array of LEDs.” The SSL has two parts: a sterilizable optical head unit, which is a disc containing electronics and LEDs, and a disposable base ring that conforms to a patient’s body contours and can be adjusted to finetune targeting of the UV-C exposure. The device is powered by three lithium-ion batteries, has timer switches for UV-C exposure duration, and features an interlock mechanism to prevent accidental activation.
Gerber chose the 235 nm wavelength after careful testing of UV-C wavelengths for maximum pathogen inactivation and minimal irritation of the patient’s skin and host tissue. “We know that 265 nm is very effective against pathogens,” said Gerber. “It affects the DNA of the pathogens, but the problem is that it also will affect the host tissue.” Gerber evaluated UV-C at 222 nm and found it, too, has drawbacks. “The problem with 222 nm is that the further you get from 265 nm, the weaker the irradiation is. Chronic wound infections usually are associated with biofilms and UV-C at 222 nm cannot really penetrate a biofilm.” The 235 nm wavelength was identified as “the sweet spot” between 265 nm and 222 nm.
According to Gerber, the device can be used to decontaminate a wound from all types of pathogens upon application, thus avoiding the 48-72 hour wait for laboratories to identify the specific pathogens affecting a patient and to determine the appropriate antibiotics. Equally as important, this UV-C technology will not contribute to the development of antibiotic resistance. And use of the SSL in operating rooms, said Gerber, “will give the surgeon an opportunity to sterilize a wound at any point during the procedure.”
Gerber plans to produce the SSL in three sizes – a three-inch diameter, a six-inch diameter and a nine- or 12-inch diameter – to allow treatment of surgical sites or chronic wound infections of various sizes. Gerber sees a need and a market for the SSL in healthcare settings, in mobile settings (visiting nurse operations, military MASH facilities) and in veterinary care. At this point, Gerber is finetuning the device and plans to seek FDA approval. He, too, is open to partnerships through financial support or expertise in the FDA approval process.
For more information on Busch’s research, email him at loris.busch@charite.de. For more information on Gerber and the Surgical Site Light, visit spectechuvc.com.
References
- R. Nussbaum, MD, Marissa J. Carter, Ph.D., Caroline E. Fife, M.D. Joan DaVanzo, Ph.D., MSW, Randall Haught, Marcia Nusgart, RPh, Donna Cartwright, MPA. “An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds.” VALUE IN HEALTH 21 (2018) p.27–32.
- Chieko Noguchi, Hironobu Koseki, Hidehiko Horiuchi, et al. “Factors contributing to airborne particle dispersal in the operating room.” July 6, 2017. BMC Surg 17, 78 (2017). https://doi.org/10.1186/s12893-017-0275-1.
- D. Chauveaux. “Preventing surgical-site infections: Measures other than antibiotics.” Orthopaedics & Traumatology: Surgery & Research 101 (2015) S77–S83. http://dx.doi.org/10.1016/j.otsr.2014.07.028.
- Antimicrobial Resistance Collaborators. “Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis.” Lancet. January 19, 2022; 399(10325): 629-655. doi: https://doi.org/10.1016/S0140-6736(21)02724-0.
- Loris Busch, Marius Kröger, Johannes Schleusener, Anna Lena Klein, Silke B. Lohan, Martin Guttmann, Cornelia M. Keck, Martina C. Meinke. “Evaluation of DNA lesions and radicals generated by a 233 nm far UV-C LED in superficial ex vivo skin wounds.” Journal of Photochemistry and Photobiology B: Biology, Volume 245, 2023, 112757, ISSN 1011-1344, https://doi.org/10.1016/j.jphotobiol.2023.112757.
- Görlitz M, Justen L, Rochette PJ, et al. “Assessing the safety of new germicidal far UV-C technologies.” Photochem Photobiol. 2023; 00: 1-20. doi:10.1111/php.13866
- Glaab, J., Lobo-Ploch, N., Cho, H.K. et al. “Skin tolerant inactivation of multiresistant pathogens using far UV-C LEDs.” Sci Rep 11, 14647 (2021). 19 July 2021. https://doi.org/10.1038/s41598-021-94070-2.
- Daniela F. Zamudio Díaz, Anna Lena Klein, Martin Guttmann, Paula Zwicker, Loris Busch, Marius Kröger, Holger Klose, Sascha Rohn, Johannes Schleusener, Martina C. Meinke. “Skin optical properties from 200 to 300 nm support far UV-C skin-safety in vivo.” Journal of Photochemistry and Photobiology B: Biology, Volume 247, 2023, 112784, ISSN 1011-1344. https://doi.org/10.1016/j.jphotobiol.2023.112784.
- Schleusener, J., Lohan, S.B., Busch, L. et al. “Irradiation of human oral mucosa by 233 nm far UV-C LEDs for the safe inactivation of nosocomial pathogens.” December 16, 2023. Sci Rep 13, 22391 (2023). https://doi.org/10.1038/s41598-023-49745-3.