By Olya Keen, University of North Carolina at Charlotte, and Laith Furatian,
Abbotsford-Mission Water and Sewer Services
UV-based advanced oxidation processes (AOPs) are relatively energy intensive compared to conventional water treatment, consuming on the order of 0.1 to 1.0 kWh/m3. 1 Thus, a rational design basis is important to avoid overdesign of AOP systems.
Role of Hydroxyl Radical Scavenging by the Background Matrix in UV AOP Process Performance
The treatment process relies on the hydroxyl radical (HO•). Since the rate of HO• reaction with most solutes approaches the diffusion limit, overall process efficiency depends on both the efficiency of HO• generation by photolysis and the aggregate rate of consumption by reactions with non-target solutes in the water matrix. In most water matrices, it is assumed that the rates of HO• generation and consumption are effectively equal, resulting in the establishment of a quasi-steady-state concentration [HO•]ss available to oxidize target compounds. Experimental observation of pseudo first-order kinetics for target compound removal provides verification that the steady-state assumption is valid.
The greater the value of [HO•]ss, the greater the removal rate of a target compound during AOP treatment. The value of [HO•]ss is directly proportional to the rate with which the source chemical (e.g., H2O2, Cl2) is converted to HO• and inversely proportional to the rate with which HO• is consumed, or “scavenged”, by the background matrix – the parameter that is colloquially known as a scavenging term (ST) or simply scavenging (Equation 1).
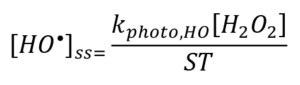 Examining Equation 1, note [HO•]ss may be increased by increasing the numerator by an increase in the dose rate of UV energy and/or the dose of H2O2 within practical limits, or by decreasing the denominator. Decreasing the denominator may be achieved by upstream processes that reduce the concentration of reactive solutes. The energy and time required to achieve a given log-removal for a target compound is inversely proportional to ST. Quantifying ST allows a determination of the feasibility of AOP treatment and, where feasible, supports a rational basis of design. The quantification of ST therefore requires repeatable and reproducible experimental methods, with adequate precision and accuracy. The development of a standardized method or protocol has been the subject of recent efforts by an IUVA Task Force co-chaired by the authors.
Examining Equation 1, note [HO•]ss may be increased by increasing the numerator by an increase in the dose rate of UV energy and/or the dose of H2O2 within practical limits, or by decreasing the denominator. Decreasing the denominator may be achieved by upstream processes that reduce the concentration of reactive solutes. The energy and time required to achieve a given log-removal for a target compound is inversely proportional to ST. Quantifying ST allows a determination of the feasibility of AOP treatment and, where feasible, supports a rational basis of design. The quantification of ST therefore requires repeatable and reproducible experimental methods, with adequate precision and accuracy. The development of a standardized method or protocol has been the subject of recent efforts by an IUVA Task Force co-chaired by the authors.
Variability of scavenging
It should be noted that ST in Equation 1 includes the HO• scavenging by both the background matrix and the oxidant added to generate HO• (e.g., H2O2 or Cl2). The major background water matrix constituents contributing to ST in typical environmental waters include dissolved organic matter and bicarbonate/carbonate. Dissolved organic matter, measured as dissolved organic carbon (DOC), ranges from < 0.1 mg/L as C in groundwater to 1 to 10 mg/L as C in rivers and lakes. 3 Total bicarbonate/carbonate in natural waters, measured as inorganic carbon and with a pH dependent distribution between the two species, ranges from 0.1 mg/L as C in pristine rainwater to up to 100 mg/L as C in groundwater. 5 In some matrices, other constituents, such as nitrite, may be significant.
Both the concentration and reactivity (reaction rate) of the background components influence the magnitude of ST. Depending on the origins of the water matrix and the oxidant dose, the background matrix can contribute as much as 95% of the overall ST, e.g., in conventional wastewater effluent 4 where most of the scavenging is represented by highly reactive effluent organic matter. In reverse osmosis (RO) permeates, often used as AOP feed in potable reuse applications, the DOC typically is < 0.5 mg/L as C. 7 Also, due to the low pH of ~5 of RO permeate, most inorganic carbon is in the form of dissolved carbon dioxide, with little in bicarbonate/carbonate form.
RO permeate thus forms an important special case for AOP consideration, given the low scavenging content of the background matrix and the consequence that most radical scavenging comes primarily from the HO• source oxidant itself and any chloramine added for membrane fouling control. In practice, chloramine concentrations in permeate can fluctuate by an order of magnitude ranging from ~0.3 to 3 mg/L. Over this range, it can contribute between 20% and 90% of the overall scavenging.
The ST can vary over time by a large factor in a given source water due to changes in both background matrix and chemical additives, which is an important consideration in application-specific reactor design.
Measurement of Scavenging
In order to properly apply ST to the design of an AOP reactor, a validated standard method should exist for reliably measuring ST in a variety of matrices of interest. An IUVA Task Force, under the direction of the authors of this article, undertook the challenge of developing a consensus-based protocol to fulfill this requirement. The Task Force has produced a draft protocol available at the IUVA website (www.iuva.org) and a spreadsheet associated with the protocol available in the IUVA member zone.
The protocol relies on the measurement of the observed pseudo first-order reaction rate constant of a probe or reference compound (adjusted for photolysis reactions in the absence of H2O2) using a quasi-collimated beam apparatus equipped with a low-pressure mercury vapor lamp. Irradiation of a well-mixed sample using a quasi-collimated beam, emitting at 254 nm wavelength, facilitates the computation of ST from measurable parameters and established constants. The ST parameter, corrected for the influence of any chemical additives such as H2O2, direct photolysis of the probe at 254 nm and any other sources of systematic error, is a property of the water matrix.
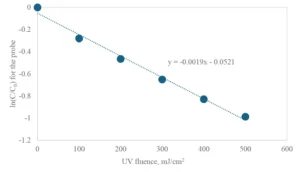
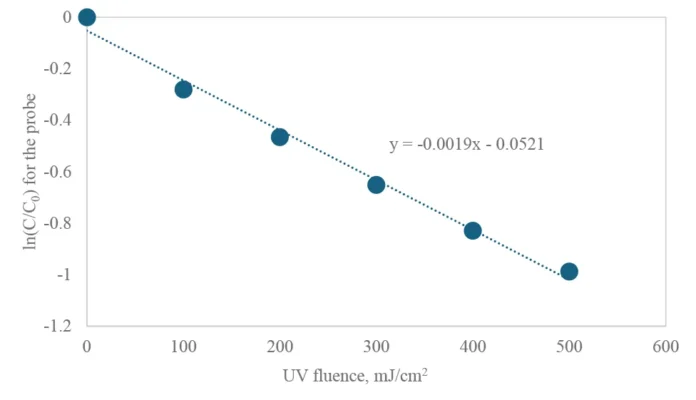
Figure 1 shows an example of the kinetic data obtained, whereby the pseudo first-order decay rate of the probe compound, calculated as the slope of the linear best fit line (0.0019 cm2/mJ), is the principal experimental parameter to be collected.
Note that the protocol remains in “draft” form pending the completion of the multi-laboratory experimental validation phase.
Future Directions
Radical probes
While the current draft protocol is based on the use of para-chlorobenzoic acid (pCBA) as the probe compound, there was a consensus among the Task Force that this choice is suboptimal. The use of pCBA to measure ST carried over to the UV AOP applications from ozone-based AOPs. 2,6 However, it is highly susceptible to direct photolysis by UV, has very low solubility and thus is a more challenging chemical to work with for the purpose of measuring ST compared to several alternatives available. The current shortlist of alternative probes consists of carbamazepine, methylene blue and sucralose. Each of these chemicals exhibits essentially non-detectable direct photolysis.
Methylene blue and sucralose have exceptional aqueous solubility for the purpose of this protocol. Methylene blue also can be measured spectrophotometrically using a visible-light-range wavelength, making this analysis more accessible to a greater number of laboratories. Carbamazepine can be analyzed with high performance liquid chromatography (HPLC) with a UV detector (such as diode array or a single wavelength detector), while sucralose requires HPLC with mass spectrometry detector (HPLC/MS). All three compounds have excellent aqueous stability.
Application of the protocol to RO permeate matrices
Experimental validation is needed to ensure that this protocol has sufficient measuring power for ST in a very low scavenging matrix typical of many AOP applications. Some of the challenges of the existing protocol are the detection limits when scavenging from the oxidant H2O2, and potentially the probe itself, greatly exceed the scavenging by the background matrix. The protocol is predicated on the validity of the steady-state assumption, which must be confirmed in such special cases.
Application of the protocol to UV/Cl2 AOP
With the rising popularity of a UV/Cl2 process, there is a need in developing a similar protocol for measuring HO• (and possibly other radicals of importance) in alternative AOP contexts. Scavenging of HO• will be the same regardless of the process used to generate HO•. However, in a UV/Cl2 process, there is a different mechanism for HO• generation and additional chlorine radicals that will be both generated, scavenged and converted to other radical forms. Either a separate protocol or guidance for use of the current protocol is the subject of future Task Force efforts.
Long-term outcomes
ST is an important parameter affecting reactor design, and typically a reactor is designed with a safety factor geared towards the highest measured ST in a set of samples collected for the water matrix. As such, a typical AOP reactor can waste a considerable amount of energy especially if the influent water is likely to experience a high fluctuation in ST over time. Because of the potential variability of ST in a given water source, there is an opportunity to optimize the energy use by AOP reactors via a reactor design adaptive to periodic or real-time scavenging measurements. Such a reactor would automatically adjust UV lamp power in response to a scavenging measurement from an in-line sensor.
Technology development in this direction would be beneficial and would need to be ultimately based on the fundamental understanding of sources and sinks of HO• for a given combination of an AOP and water matrix composition.
Establishment of a consensus-based standard protocol for the quantification of ST is intended to support the rational and optimal design of AOP systems in terms of treatment performance and energy efficiency.
References
- Howe, K.J., Hand, D.W., Crittenden, J.C., Trussell, R.R. and Tchobanoglous, G., 2012. Principles of water treatment. John Wiley & Sons.
- Thurman, E.M., 1985. Organic geochemistry of natural waters. Martinus Nijhoff/Dr W. Junk Publishers.
- Stumm, W. and Morgan, J.J., 1996. Aquatic chemistry: chemical equilibria and rates in natural waters. John Wiley & Sons.
- Keen, O.S., McKay, G., Mezyk, S.P., Linden, K.G. and Rosario-Ortiz, F.L. 2014. Identifying the factors that influence the reactivity of effluent organic matter with hydroxyl radicals. Water Res 50, 408-419.
- Mackey, E., Hofmann, R., Festger, A. and Vanyo, C. 2022. UV-Chlorine AOP in potable reuse: A guidance manual to assessment and implementation. Water Research Foundation.
- Elovitz, M.S. and von Gunten, U., 1999. Hydroxyl radical/ozone ratios during ozonation processes. I. The Rct concept. Ozone: Science & Engineering 21(3), 239-260.
- Rosenfeldt, E.J. and Linden, K.G. 2007. The ROH,UV concept to characterize and the model UV/H2O2 process in natural waters. Environmental Science & Technology 41(7), 2548-2553.