By Troy Cowan, IUVA Healthcare / UV Working Group facilitator
Congratulations to our friends and colleagues at ASHRAE for a well-planned and well-executed ASHRAE Winter Conference in Chicago, where Dr. Rick Martinello 1 and I recently had the privilege of representing IUVA, thanks to our IUVA/ASHRAE MOU. 2 Kudos to the ASHRAE staff for their wonderful service and assistance, as well as their excellent work.

Prime motivation for attending was to learn more about ASHRAE Standard 241-2023, “Control of Infectious Aerosols” (aka, ASHRAE 241, Image 1) 3 and related ASHRAE standards (e.g., 62.1 & 62.2, 170, 185.1, et al). With this objective in mind, I attended several sessions related to Technical Committee 2.9 (TC 2.9) activities, led by TC 2.9 Chair and IUVA Board Member, Ashish Mathur (Well done, Ashish!). I also attended classes dealing with the basics of design and implementation of ASHRAE 241-compliant systems, one of which was taught by Drs. William Bahnfleth and Marwa Zataari on “Understanding ASHRAE Standard 241” 4 – a good course and well worth the investment.
In his part of the course presentation, Dr. Bahnfleth did an excellent job of making the case for how changes in Indoor Air Quality (IAQ) standards are needed “to address airborne infection transmission risk,” because “existing standards explicitly exclude infection risk mitigation as a criterion.” This is the main driver for implementation of ASHRAE 241 – to control infectious aerosols. 5
In this context, Dr. Bahnfleth emphasized ASHRAE’s focus on engineering controls as an effective means to isolate people from potential infectious hazards (Image 2). His coverage of germicidal ultraviolet (GUV) technology as one of those engineering controls was outstanding, highlighting that it is a very effective method for inactivating microbes, “studied and applied for more than 100 years,” part of a multimodal approach to managing risk from infectious aerosols. 6
He addressed the importance of knowing the UV ‘deactivation constant’ (aka, k value), how it varies based on the target microbe and the GUV wavelength employed. As examples, he presented k values for Anthrax and MHV Coronavirus, showing a 60+ fold difference between them when using 253.7 nm GUV, trying to achieve the same level of inactivation. In the presentation, Dr. Bahnfleth affirmed that when comparing deactivation dosages between bugs, if one ‘bug’ requires a bigger dose over another, that stays the same, regardless of wavelength. 7 This may enable relative bug-to-bug efficacy comparisons based on deactivation curves.
The course went on to explain the concept of Equivalent Clean Airflow (ECA) – how it’s derived and how it’s used as the key factor in assessing how much infection risk mitigation will result from the various engineering control technologies being employed. Dr. Bahnfleth explained how the required amount of ECA varies by type of facility, planned occupancy levels and facility’s use (e.g., commercial/retail facilities vs. educational vs. healthcare, etc.). He also covered how to calculate the blended ECA flows when more than one engineering control technology is used (e.g., outside ventilation and filtration and GUV in combination).

Dr. Bahnfleth further explained that to calculate the contribution of GUV devices in ECA flows, ASHRAE 241 requires the GUV system to be tested for effectiveness against one particular ‘challenge’ microbe – the nonenveloped bacteriophage MS2 (Image 3). 8 A commonly used microbial test surrogate, MS2 is “one of the smallest microbes known…” 9, with its viron measuring ~27 nm in diameter. 10 As such, it presents a difficult challenge for GUV inactivation, due to its small size and high GUV resistance 11, making it harder to hit with sufficient flux to cause the requisite RNA damage.
While the UV water disinfection community has long used MS2, its small size and GUV resistance has been the topic of many discussions inside IUVA’s air and surface treatment circles. There, concerns often were raised, e.g., that MS2 was ‘too difficult to inactivate’ compared to most infectious pathogens, that using it as the standard would cause ‘over-treatment of the space, wasting energy’ and that the over-treatment would result in ‘producing more ozone and other byproducts’ than necessary, etc. In contrast, Dr. Martinello shared in conversation that MS2 does have an upside – it’s easily handled in BSL-1 test facilities, making testing more accessible and less costly compared to BSL-2 pathogens. Also, it is a well-recognized and accepted infectious pathogen surrogate (shared with Dr. Martinello’s permission). 12
In all of the discussions about MS2, I noticed that the word ‘surrogate’ kept coming up. Even ASHRAE 241 mentions it in Normative Appendix A, Item A1.3.2:
“Bacteriophage MS2 (host Escherichia coli) is chosen because of its established properties as a relatively safe and conservative infectious pathogen viral surrogate… Air cleaner manufacturers are encouraged … to determine more specific performance claims against known infectious pathogens that are outside the scope of this standard.” 13
So, in this soon-to-be ANSI standard, ASHRAE 241 acknowledges MS2 as only a surrogate and, as such, it is not an infectious pathogen. Further, it infers that use of known infectious pathogens are outside of ASHRAE 241’s scope. These two points bring up some interesting questions:
- Does the medical community concur that MS2 is only a surrogate and not an infectious pathogen? If so…
- Would a citation in a GUV device label, reporting results from an ASHRAE 241-certified test showing measurable reductions in MS2 therefore not be a medical claim?
In discussions with Dr. Martinello, he concurred that these appeared to be the correct conclusions, but readily acknowledged that FDA would be the final authority. If correct, this could lead to two potential ways that factual statements (aka, claims) could be made about MS2 reductions without making a medical claim:
- Option A: Test the GUV device(s) directly against MS2, using ASHRAE 241 testing protocols 14, citing the MS2 reduction results in the labels, much like the traditional method for validating UV-C water treatment systems using EPA Guidance 15, or
- Option B: Measure the device’s specific GUV output using IES/IUVA LM standards 16, and from these measurements, infer the device could have
the capability of reducing MS2 up to an estimated amount, derived using an industry standard MS2 inactivation curve 17, 18 and the IES/IUVA LM-measured output.
Option A is very straightforward but potentially lengthy and costly due to the number of samples and amount of BSL-1 lab work required. Option B is equally straightforward but much cheaper and quicker as it requires no BSL-1 capabilities, relying instead on GUV standard photonic measurements, looking up the results on an industry standard MS2 inactivation curve.
But therein lies the rub: There currently are no “industry standard” MS2 inactivation curves. That’s, in part, because there is no “industry standard” inactivation curve testing protocol.
There are multiple inactivation protocols for water disinfection curves, some of which have been in place for more than 20 years (e.g., one done by IUVA veterans Drs. Jim Bolton and Karl Linden in 2003 19, and EPA’s 2006 guidance 20). These are focused on water testing in reactors and petri dishes, not aerosol samplers or surface coupons. While these are long-standing, highly regarded practices and guidelines, they have yet to be memorialized as industry consensus standards or regulatory requirements, resulting in a lack of consistency in their application, especially when it comes to their adaptation to air and surface studies.
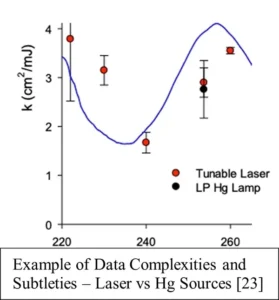
As a recent example, a highly sophisticated protocol was employed in a SARS-CoV-2 study done by Battelle National Biodefense Institute for the US Department of Homeland Security (DHS), the National Institute of Standards and Technology (NIST), US Department of Commerce and the Lyles School of Civil Engineering, Purdue University. 21 It involved tunable GUV lasers and standard 253.7 nm Hg sources, specialized exposure chambers and calibrated radiometers. (An example of its results is shown in Image 4. 22) In its methodology, it referenced the Bolton/Linden protocol regarding UV water reflectivity only (re: endnote [46]); EPA 2006 Guidance was not addressed. 23
Note: As this study solely addressed infectious SARS-CoV-2, any mention of it in GUV device literature has the potential of being deemed a medical claim by either FDA, EPA or both, thereby invoking their regulations, opening up vulnerabilities to possible enforcement actions.
There are other activities going on involving inactivation constant protocols:
- Drs. Jim Bolton and Madjid Mohseni are leading an IUVA Task Force to populate and maintain a Microorganism UV Sensitivity Database, possibly involving NIST.
- Drs. Ernest Blatchley and Sun Zhe are leading a TRAST-initiated effort to define one or more protocols that can be used to define the intrinsic kinetics of UV-based inactivation of aerosolized microbes and viruses.
- Dr. Joy Dunkers is working on more accurate methodologies for determining efficacy in the surface testing proposed under ASHRAE 185.4.
- Under ASHRAE TC 2.9, led by Dr Ashish Mathur, a research procurement is underway to conduct a comprehensive literature review and develop a database of UV rate constants.
And, there may be others but, so far, none are for an industry standard for the protocol. What if, as a collaboration between the above efforts, we could develop an industry consensus standard protocol (i.e., ANSI standard) for developing deactivation curves that bridged water, air and surfaces? Then, could we develop/compile a single, certified set of deactivation curves for MS2 (water, air and surface)? And wouldn’t that be citable across the board without there being a medical claim? And, if comparable curves also were developed using the same protocol for SARS-CoV-2, Influenza, C. diff, MRSA, Candida auris, et al, couldn’t the healthcare community and public space managers do their own comparisons of the certified results and draw their own conclusions?
We need the protocol, and we need these deactivation curves, to help us get the message out that GUV is credible, and it works. To formalize the protocol into an industry consensus standard requires industry collaboration. Then, to get these deactivation curves requires lab research and analysis resources formulated into focused projects. Interested? Let me know. Let’s do this. It’s all about saving lives.
References
- Richard A. Martinello, M.D., Professor, Yale School of Medicine, and IUVA Board member
- ASHRAE & IUVA Memorandum of Understanding, signed 21 April 2021
- ASHRAE Standard 241-2023, “Control of Infectious Aerosols,” ASHRAE, 7 July 2023, https://www.ashrae.org/about/news/2023/ashrae-publishes-standard-241-control-of-infectious-aerosols
- Bahnfleth and Zataari, “Understanding ASHRAE Standard 241, Control of Infectious Aerosols Background, Overview, and Key Requirements, an ASHRAE ALI course, https://www.ashrae.org/professional-development/all-instructor-led-training/catalog-of-instructor-led-training/understanding-ashrae-standard-241-control-of-infectious-aerosols-background-overview-and-key-requirements
- Ibid
- NIOSH, “Hierarchy of Controls,” an Image, https://www.cdc.gov/niosh/topics/hierarchy/default.html
- See Ref 2 (Bahnfleth and Zataari), shown above
- Óttar Rolfsson, Stefani Middleton, et al, “Direct Evidence for Packaging Signal-Mediated Assembly of Bacteriophage MS2,” Journal of Molecular Biology, Volume 428, Issue 2, Part B, 29 January 2016, Pages 431-448, https://www.sciencedirect.com/science/article/pii/S0022283615006762?ref=pdf_download&fr=RR-2&rr=84d50af71efa29b6
- Fiers, W., Contreras, R., Duerinck, F. et al. Complete nucleotide sequence of bacteriophage MS2 RNA: primary and secondary structure of the replicase gene. Nature 260, 500–507 (1976). https://en.wikipedia.org/wiki/Bacteriophage_MS2#cite_note-Fiers-Ref#3
- Strauss JH, Sinsheimer RL (July 1963). “Purification and properties of bacteriophage MS2 and of its ribonucleic acid.” Journal of Molecular Biology. 7: 43–54. doi:10.1016/S0022-2836(63)80017-0. PMID 13978804
https://en.wikipedia.org/wiki/Bacteriophage_MS2#cite_note-Straus-Ref#4 - Baldasso, Lubarsky, et al, “UV-C inactivation of MS2-phage in drinking water – Modelling and field testing,” Water Research, Volume 203, 2021, 117496, ISSN 0043-1354, https://doi.org/10.1016/j.watres.2021.117496
- Beck, Rodriguez, Hawkins, Hargy, Larason, and Linden, “Comparison of UV-Induced Inactivation and RNA Damage in MS2 Phage across the Germicidal UV Spectrum,” ASM Journals/Applied and Environmental Microbiology, Vol. 82, No. 5, 19 February 2016, https://journals.asm.org/doi/10.1128/aem.02773-15
- See Ref 1 (ASHRAE Standard 241-2023, shown above), pg. 25
- See Ref 1 (ASHRAE Standard 241-2023, shown above), Normative Appendix A
- EPA 815-R-06-007, “Ultraviolet Disinfection Guidance Manual for the Final Long Term 2 Enhanced Surface Water Treatment Rule,” EPA Office of Water (4601), November 2006, ‘Appendix C. Collimated Beam Testing to Develop a UV Dose-response Curve’, https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=600006T3.txt
- Miller, NIST, Yuqin Zong, NIST, and Jeff Hulett, Vektrex, “New Optical Metrology and Documentary Standards for GUV Radiation Sources,” UV Solutions, 2023 Quarter 1, https://uvsolutionsmag.com/articles/2023/new-optical-metrology-and-documentary-standards-for-guv-radiation-sources/
- Masjoudi, Mohseni, and Bolton, “Sensitivity of Bacteria, Protozoa, Viruses and Other Microorganisms to Ultraviolet Radiation,” August 20, 2021, https://doi.org/10.6028/jres.126.021
- See Ref 11 (Beck, et al, “Comparison of UV-Induced Inactivation…”)
- Bolton, Linden, “Standardization of Methods for Fluence (UV Dose) Determination in Bench-Scale UV Experiments,” DOI: 10.1061/(ASCE)0733-9372 (2003), 129:3(209), https://www.academia.edu/48266086/
- See Ref 17 (EPA 815-R-06-007,…)
- Schuit, Larason, Krause, Green, et al, “SARS-CoV-2 inactivation by ultraviolet radiation and visible light is dependent on wavelength and sample matrix,” J Photochem Photobiol B. 2022 Aug; 233: 112503, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9221687/
- Data from: Schuit, Larason, et al, “SARS-CoV-2 inactivation by ultraviolet radiation and visible light is dependent on wavelength and sample matrix,” Journal of Photochemistry & Photobiology, B: Biology, 233, 112503. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9221687/pdf/main.pdf
- Ibid.