Originally written by Dr. Eugene I. Gordon Ph.D., IEEE Fellow and Edison Medal Recipient, and Peter E. Gordon MSEE, in April 2012. Updated in July 2023 by Peter E. Gordon for the benefit of the UV-C and Far UV solution design and global user community. Provisional Patent has been filed.
Editor’s Note: Part 1 was published in Issue 3 2023 of UV Solutions, www.uvsolutionsmag.com.
Part I gave detail on germination that is essential reading to get into the weeds of the germination measurement technique using dipicolinic acid (DPA) as an alternative to petri dish measurement. Furthermore, Part 1 describes a method that now must be examined to overcome potential roadblocks to implementation, such as false readings. Hence, Part 2 of the article commences with a dive into the endospore inactivation dynamics pool in order to work through demonstrating that the method is repeatable, reproducible and universal.
The Fly in the Ointment: Complications
Given that UV-C acts by damaging a variety of DNA molecules in the endospore, including one that controls mitosis (reproduction of vegetated bacteria), and also given that Far UV acts by multiple damaging mechanisms 1 which may affect mitosis in a more complicated way if the particular trigger DNA for mitosis is damaged but not demobilized within the endospore by either type of radiation, during an infection-prevention surface application or room or in duct air treatment deployment where the GUVeA BI is utilized, then fully activated bacterium within the bio-photonic brew may not reproduce and are not viable, but could still throw the results off. That is, acknowledging that disinfection is imperfect since it does not achieve sterilization, the results would be that many of the endospores of the initial population still will germinate when exposed to nutrient but never achieve a viable, activated, vegetated state in which they can replicate and colonize. In another case, the endospore may enter a super-dormant or zombie state, where the reproductive mechanism has not been damaged but the ability of an endospore to activate and germinate has been impaired, creating endospores in limbo that could awaken, rally and manifest germination. Controlling conditions for germination, not only in relation to activation but also to encourage and motivate homogenous germination, rather than lumpiness due to delays, is imperative. 2
In any case, many more germinating endospores are manifest than there are bacteria that can fully and completely activate and become vegetated bacteria capable of reproducing and forming colonies. Hence, germinating endospores are thus described as false survival positives and, as a consequence, a skeptic could conclude and assert that counting germinations is not equivalent to counting colonies. Hence, the germination-counting technique could be dismissed as inaccurate and nominally useful.
In fact, this conclusion would be incorrect because the number of germinating endospores vs. number of colonies counted in a petri dish as a function of UV-C or Far-UV dose can be shown to be measured and to be precisely related at any given dose value. And once the germination rate is determined, the colony forming rate is known. Thus, there can be no false positives if one makes the necessary corrections and calibrations. Counting germinations then is demonstrated to be a legitimate alternative to counting colonies, and significantly quicker. More information follows.
Insights
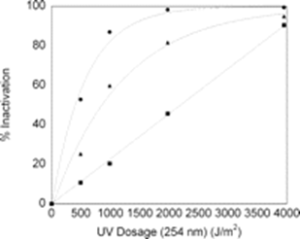
In a paper by Shafaat and Ponce, 3 the DPA-based photo luminescent technique is described and used to measure endospore germination by UV-C. Figure 4 is a reproduction of original Figure 5 in the Shafaat and Ponce paper. When examined, it undermines the story of false positives as it relates to use of DPA photoluminescence for determining inactivation rate vs. UV-C dose of a selected test endospore (Bacillus subtilis). The figure summarizes measurement of the performance of the UV-C disinfection system using petri dish colonization, EVA (which is the DPA photoluminescence technique) and phase contrast microscope. The phase contrast microscope measurement is not relevant and won’t be discussed here.
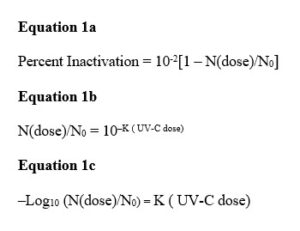
The measurement data on the number of pathogens forming colonies, N(dose), vs. UV-C dose fits closely the expected percent inactivation exponential curve given by Equations 1a and 1b, in which No is the control or starting number of pathogens and K is a value determined from the data producing the best fit. The key point is that the same exponential curve also fits the DPA-measured germination data, but the constant K differs for germinations vs. colony forming. K is smaller for germinations, which require a larger dose for a given percentage reduction relative to the control value. This subtle point was not discussed by Shafaat-Ponce, but it is critical.
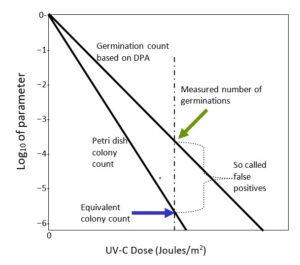
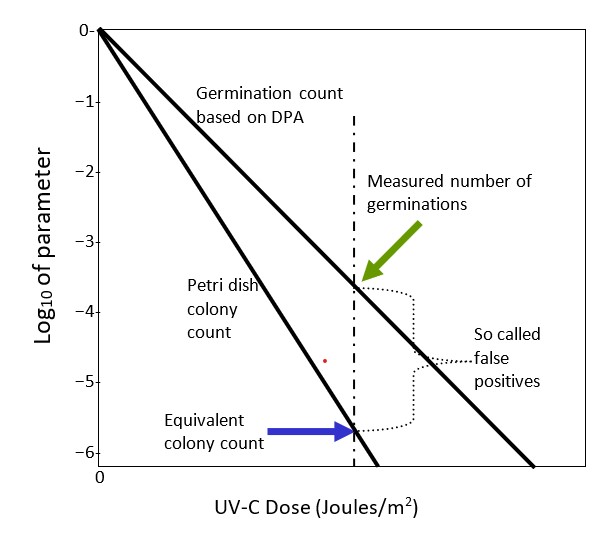
At any given value of UV-C dose, the vertical difference between the value on the colony forming units (CFU) curve and 100% is the number of reproducing, colony-forming survivors. It is critical to note again that both the percent inactivated curves for CFU and EVA (the DPA technique) are exponential and differ only in terms of the UV-C dose parameter, the inactivation parameter K in Equation 1C. K from either approach readily is determined. It means that the CFU curve can be determined from the EVA curve and explains why a measurement of one value on the EVA dose curve allows determination of the number of colonies that would result.
It is precisely this reason that the concept of false positive is fallacious and why the DPA technique is equivalent to CFU measurements. It illustrates why equating colony forming positives with germination counts is incorrect and misleading. Again note, CFU is the gold standard for measuring inactivation of pathogens. If pathogens do not form colonies in a petri dish or equivalent, they have been inactivated, cannot multiply and cannot cause infections, even if they survive as sterile vegetated bacteria.
As illustrated in the paper, at approximately 1,500 Joules/meter2, the potential CFU inactivation is ≈ 90% (the D90 dose criterion), although the units of the graph seem to be incorrect. The actual D90 value for bacillus subtilis endospores, depending on strain and as shown in multiple papers, is about 150 Joules/meter2. 4,5 It could be that the authors made a typographical error, or they are not measuring the UV-C intensity directly incident on the endospores.
Regardless of the values and units, the key insight is that once the two values of K (let’s call them Kcc and Kger) are known and validated, the unit can determine colony forming data from the DPA photoluminescent germination measurements. Optimism grows that the technique works, and graphical representation should be straightforward.
Affirmation: Colony Count and Germination Data vs. Applied Dose
From Figure 4, it is clear that as the UV-C dose increases the percentage of surviving, colonizing, vegetated bacteria and the numbers of germinating endospores decreases exponentially with increasing UV-C dose. The increased dose not only damages the trigger DNA molecule controlling mitosis but damages the ability to germinate. The two damage mechanisms are independent, although both track increased dose. Figure 5 is a representation of the Shafaat and Ponce colony count and the germination data, N, plotted on the conventional -log10 N/No vertical axis vs. the applied UV-C dose on the horizontal axis. Zero on the vertical colony count scale is the control value; N = N0. Colony count data on such a plot should fall on a straight line with a negative (Kcc) slope, as indicated by Equation 1c, which is consistent with the exponential Shafaat and Ponce data, as reproduced in Figure 4. Furthermore, within the Shafaat and Ponce dose range, there is no sign of clumping, which would cause a distinct tail and a kink in the linear curve.
Similarly, one would expect that the germination data would behave similarly with a less steep (Kger) slope value. In fact, it does, as discussed, on the Shafaat and Ponce plot. The slope values have a distinguishable ratio for CFU vs. germination. For a given test pathogen such as Bacillus subtilis, the petri dish colony count and the DPA measured germination count are determined in separate experiments. However, germination data readily and accurately translates into colony count data. The key point is that the germination curve is readily translated into the typical colony count curve and germination can substitute for colonization as a precise measure of failure of inactivation. Hence, the false positive issue is resolved.
The realized hardware solution should be able to do the faster EVA germination measurement and accurately correct the data based on experience gained by doing comparative experiments as a function of UV-C dose. Put another way, the solution can correct the EVA measurement to provide the true colony inactivation rate. This would give a good approximation of the remaining, viable, vegetated bacteria, and additional proportionality factor (Kcc / Kger)) confirmation experiments by UV-C industry microbiologists would be desirable and welcome. It is important to note that this approach will not operate in the case of spore clumping in the sample. But, from experience, avoiding clumping in the sample is an exercise with a known protocol practiced widely in microbiology testing labs. 6 Now, onto realizing hardware so a GUVeA can be applied to a plethora of UV-C disinfection applications.
Next Steps and a Path Forward
The measurement principles of an ultrafast (effectively ‘instant’) BI for UV-C and Far-UV disinfection, the GUVeA, have been discussed and feasibility indubitably demonstrated. Constructing the BI of quartz, TEFLON® AF or TOPAS® COC, all of which essentially are UV-C and Far-UV transparent, would be straightforward. Medical-grade capsules based on these materials exist today. 7 Sterile insertion of the appropriate nutrient would initiate germination and allow measurement of the number germinating. As an example, autoclave BI, discussed in Part 1, simply rely on a thumb mechanism depressing a cap to trigger media mixing and verification. 8
Design and demonstration of a fast electronic reader for detecting germination are not challenging and could be inexpensive to produce. Today, bio-photonic reading is commonplace in wearable devices, which means that they could be available in small form factor and be inexpensive. Since, currently, 254 nm low-pressure lamps and xenon lamps dominate UV-C generation, with Far-UV lamps following in the near future for full-room illumination, the real-time, ultrafast BI tuned to these sources would provide a unique and highly desirable capability currently lacking in practice using an installed solution base as alpha adopters. One could foresee that soon-to-be regulated disinfection box “autoclaves” 9 also could utilize such a sensor. Upper-room air would require insight into suspension of the GUVeA capsule, midair, so designers, implementors, installers and operators of upper-room air solutions, seeking UL 8802 certification and follow evolving GPC 37 guidelines, explicitly demonstrating efficacy, take fully advantage of the innovation. 10 In-duct capability would require access points. Potentially, Part 3 of this article could explore its use in smaller drinking water or critical wastewater treatment systems to verify efficacy on a weekly or monthly basis. One could imagine a flotilla of capsules inserted into a parallel channel that replicates the current operating conditions, floating along, and then retrieved after exposure in the reactor. The same concept could apply to ballast water. Its unique use for assuring efficacy in drinking water and, particularly, UV-C plus AOP wastewater treatment reactors requires waterproofing and further investigation into the inactivation mechanisms due to complex chemistries. 11
For completeness, it is important to note that biological indicators are Class II devices subject to premarket clearance (510(k)) requirements. FDA has no required performance standards for biological indicators. Biological indicators may be claimed equivalent to: (1) a related classified device that was in interstate commerce before 1976; and not requiring clearance or (2) a device cleared through the 510(k) process and currently legally marketed. 12 The first possibility doesn’t apply in this case, but one could argue that the second does. Demonstrating equivalence should be quick and inexpensive.
In summary, early publications showed the role of DPA as the chemical basis for a unique and novel biological indicator. Its viability had been underappreciated, and this still currently is the case since there is no commercialized solution available. However, it was the aspiration of this article to motivate serious investigation into the premise and to spur investment into realization. It is justified and imperative to the scientific and financial well-being of the GUV eco-systems to pursue such capability to imbue trust and confidence in the effectiveness of GUV within the validation, implementation and oversight communities.
Dr. Eugene I. Gordon Ph.D, IEEE Fellow and Edison Metal Recipient, was co-inventor of the Argon Ion laser (applied to treating diabetic retinopathy), the CCD Imaging device (enabling fax machines and night vision googles), and Video Conferencing (The PictuePhone was first used during the Moon Landings).
Peter E. Gordon is director of business development, CudoForm Tech, Senko Advanced Components, Inc. and is primarily focused on Photonic IC Optical I/O to Fiber Optic connectivity solutions for next-generation Ethernet Switches in Data Centers and AI chip-to-chip, node-to-node in high-speed computers. He is a member of EPIC, Optica, the IEEE, ASHRAE, the UL8802 standards group and the I UV-A, and an elected member of the Board of Education in Weston, Connecticut. For more information, email peter.gordon@senko.com for Senko Advanced Components, Inc. or pgordon051163@gmail.com for LiTeProducts LLC.
References
- Narita, K. et al. 222-nm UV-C inactivates a wide spectrum of microbial pathogens. J Hosp Infect (2020)
- Warriner et al., Inactivation of Bacillus subtilis Spores on Aluminum and Polyethylene Preformed Cartons by UV-Excimer Laser Irradiation, Journal of Food Protection 63(6):753-7, June 2000 and private conversation
- Hannah S. Shafaat and Adrian Ponce, Applications of a Rapid Endospore Viability Assay for Monitoring UV Inactivation and Characterizing Arctic Ice Cores, APPLIED AND ENVIRONMENTAL MICROBIOLOGY, Oct. 2006, p. 6808–6814 Vol. 72, No. 10
- Wladyslaw Kowalski, Ultraviolet Germicidal Irradiation Handbook UVGI for Air and Surface Disinfection, Springer, 2009
- Thomas P. Coohill and Jose-Luis Sagripanti, Overview of the Inactivation by 254 nm Ultraviolet Radiation of Bacteria with Particular Relevance to Biodefense, Photochemistry and Photobiology, 2008, 84: 1084–1090
- Yung et al., Quantitative and Fast Sterility Assurance Testing of Surfaces by Enumeration of Germinable Endospores, Sci Rep. 2020 Jan 16;10(1):431
- https://topas.com/sites/default/files/TOPAS-Medical-03.08.21.pdf
- www.getinge.com/dam/hospital/documents/english/mcv00058546_assured_overview_0910_brochure_5-en-us.pdf\
- Guideline R2540 UV-C disinfection of non-critical and semi-critical medical devices. 1 jun. 2023
- https://uvsolutionsmag.com/articles/2022/gpc-37-guidelines-for-the-application-of-upper-room-germicidal-ultraviolet-uv-c-devices-to-control-transmission-of-airborne-pathogens-a-preview/
- Guo et. al., UV/Chlorine Process: An Efficient Advanced Oxidation Process with Multiple Radicals and Functions in Water Treatment, Acc. Chem. Res. 2022, 55, 3, 286–297
- http://www.fda.gov/OHRMS/DOCKETS/98fr/010193gd.pdf