By Eirini Vitzilaiou, Ph.D., Postdoctoral researcher, University of Copenhagen, Department of Food Science, Section of Microbiology and Fermentation
Background
Food processing water reuse
The food processing industry needs large amounts of water daily for cleaning and transportation purposes, as well as the different food processing steps from the raw materials to the final products. However, drinking water intake and, even more, wastewater discharge have a high cost, and therefore the food industry is eagerly focusing on reducing its water footprint. One of the common ways to do so is by reconditioning the water from the different food processing steps to reuse it within the industrial facilities for direct, indirect, or non-food-contact purposes.
Based on each food production and processing step, the food processing water may have high or low microbial population levels. Microbial contaminants in the food processing water can lead to spoilage of the final products through water reuse, reducing product quality and shelf-life, and increasing food waste. Moreover, microbial growth within the water distribution and storage system can lead to biofilm formation on processing equipment, decreasing process efficiency. Finally, pathogenic microorganisms may be found in the food processing water, putting at risk the safety of the final products. Therefore, the application of treatments to eliminate or reduce the microbial contaminants in the food processing to acceptable levels before water reuse is essential. This procedure is called reconditioning1 and UV-C light disinfection, employing low-pressure mercury lamps, is one of the common technologies used for food processing water reconditioning, usually in combination with membrane filtration2,3.
The tailing effect
The UV inactivation kinetics curve follows a first-order disinfection model, where there is a linear relationship between the log10 inactivation and the UV dose4,5. However, a decrease in the inactivation rate is often observed at the longer exposure times or higher UV doses after the largest part of the initial microbial population has been inactivated. This deviation is called tailing. The tailing phenomenon has been documented by several studies in aqueous suspensions of bacteria, bacterial spores, yeasts and phages under static and flow-through UV reactors employing mercury lamps and Light-Emitting-Diodes (LEDs) at different wavelengths6-14. Among the several hypotheses behind the tailing effect, in this article, we will focus on microbial aggregation leading to light-shielding.
Microbial aggregation and light-shielding
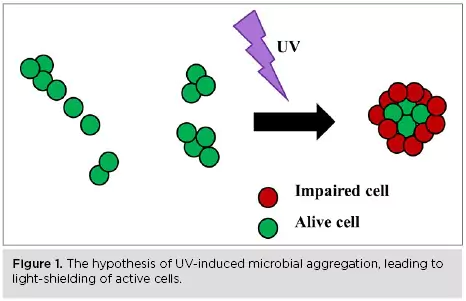
Microbial aggregates are three-dimensional cell clusters, with a structure similar to a biofilm, that float freely into a solution15-18. Chemical disinfectants, antibiotics or UV light may not penetrate the aggregates, and therefore the microbial cells in the center are protected during disinfection and remain active6,8,17-19. Light-shielding is specifically the phenomenon when the UV light does not penetrate the center of the aggregate during UV disinfection (Figure 1).
Microbial aggregation: Can it be UV-induced?
The ability of the microbial cells to aggregate may be an inherent strain characteristic and /or induced by several factors. For instance, a high probability of cells to collide with each other can result in microbial aggregation. The high microbial population levels and the use of UV reactors, inside which the treated solution is mixed in bulk and under high flow rate, increase the probability of cell collision during UV disinfection10,19,20.
Moreover, the pH and ionic strength of the suspension matrix, as well as the physiological state and viability of the cells, may further affect the cell dispersal state21. Specifically, it has been found that the cell membrane surface charge is becoming less negative when the cells are under stress or have low viability, and thereby the cells may more readily attach. UV light also may induce aggregation by reducing cell viability or by directly interacting with the cell membrane surface19,21.
The impact of microbial aggregation
UV radiation has no residual action; therefore, if cells within the aggregates survive UV treatment, microbial growth can take place during storage of the reconditioned water, increasing the possibility for cross-contamination of products and equipment surfaces, and thereby putting at risk the quality and safety of the final product. Although there is wide research on the efficiency of UV light for drinking water treatment, studies on UV disinfection for food processing water are limited2,20,22,23. The aim of this study was to a) investigate the impact of bacterial cell aggregation on UV inactivation kinetics and b) assess whether aggregation can be UV induced. For this purpose, bacterial strains, naturally aggregating and non-aggregating, were exposed to increasing UV-C doses, and the Total Viable Counts (TVC) and particle area were compared between UV-treated and the corresponding control samples.
Experimental design
 Strain selection
Strain selection
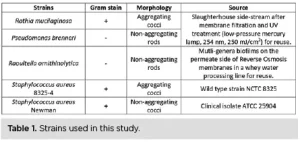
Five strains were selected for the UV exposure experiments, three of which were isolated from food processing water reuse lines after membrane filtration and/or UV treatment: Raoultella ornithinolytica (R. ornithinolytica), Pseudomonas brenneri (P. brenneri) and Rothia mucilaginosa (R. mucilaginosa). To compare UV inactivation kinetics and bacterial aggregation within and among species, two culture-collection strains belonging to Staphylococcus aureus (S. aureus) also were included. One strain was naturally aggregating and the other was not17 (Table 1). One more scenario was tested in which the aggregates of the aggregating S. aureus strain were broken to assess whether UV efficiency was increasing.
 UV exposure
UV exposure
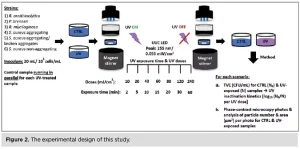
The UV LED device PearlLab Beam™ (Aquisense Technologies/Erlanger, Kentucky, USA) was used to expose the samples to UV-C light at 255 nm, a wavelength close to that of the low-pressure mercury lamp (254 nm), used in the industrial set-up. The experimental protocol was structured with the technical support of the UV LED manufacturer. In this study, for the first time, a control sample was used for each UV-exposed sample and for each UV dose, running in parallel on an identical UV LED set-up with the shutter and UV button turned off to differentiate between UV-induced and time-induced aggregation. Both samples were handled and analyzed in the same way (Figure 2).
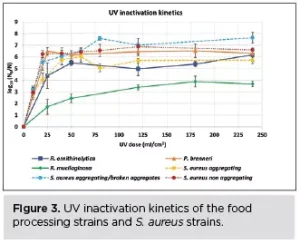 UV inactivation kinetics
UV inactivation kinetics
The log10 inactivation was calculated as log10 (N0/N), where N0 is the average TVC (CFU/mL) of the control sample and N is the average TVC (CFU/mL) of the UV-exposed sample. The UV inactivation kinetics were determined as the log10 inactivation plotted against the corresponding UV dose24,25.
Phase-contrast microscopy
The increase or decrease in particle area from the control to the UV-exposed samples was determined for each UV dose by capturing photos from several different spots of control and UV-exposed samples when placed on microscope slides. For this purpose, a phase-contrast microscope and an imaging source were used: The photos were analyzed using the software Image J, which counts the number and the area (μm2) of the particles per photo.
Results and Discussion
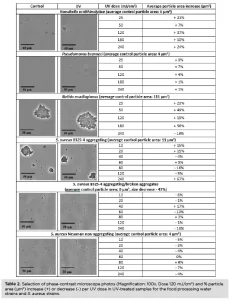 R. mucilaginosa was the most aggregating strain, with a particle area of 111 μm2 before UV exposure (Table 2), and the most UV-tolerant among the strains tested (Figure 3). The non-aggregating S. aureus and P. brenneri strains were the least aggregating before UV treatment, with a particle area of 4 μm2 (Table 2) and the least UV-tolerant (Figure 3). S. aureus aggregating strain exhibited a higher UV tolerance than the non-aggregating strain, but when the aggregates were broken, the UV tolerance became similar to that of the non-aggregating (Figure 3). The tailing phenomenon was observed for all the strains in this study, aggregating and non-aggregating. However, the tailing onset took place earlier for the least UV tolerant and later for the most UV tolerant strains (Figure 3).
R. mucilaginosa was the most aggregating strain, with a particle area of 111 μm2 before UV exposure (Table 2), and the most UV-tolerant among the strains tested (Figure 3). The non-aggregating S. aureus and P. brenneri strains were the least aggregating before UV treatment, with a particle area of 4 μm2 (Table 2) and the least UV-tolerant (Figure 3). S. aureus aggregating strain exhibited a higher UV tolerance than the non-aggregating strain, but when the aggregates were broken, the UV tolerance became similar to that of the non-aggregating (Figure 3). The tailing phenomenon was observed for all the strains in this study, aggregating and non-aggregating. However, the tailing onset took place earlier for the least UV tolerant and later for the most UV tolerant strains (Figure 3).
UV light increased significantly the particle area in all the food processing water strains, whether aggregating or not, from the lowest UV dose of 25 mJ/cm2 (R. ornithinolytica: + 21%, P. brenneri: + 3%, R. mucilaginosa: + 23%) (Table 2), showing that UV induces aggregation, in accordance with previous studies6,19,26. The particle area difference between control and UV-exposed samples did not further significantly increase by increasing the UV exposure time, indicating that any impact of the UV light on the cells leading to aggregation had been already exerted at the first UV dose applied.
Interestingly, the first significant increase in particle area coincided with the first high log10 reduction for all the food processing water strains at 25 mJ/cm2: (R. ornithinolytica: 4.5 log10 reduction, P. brenneri: 6.5 log10 reduction, R. mucilaginosa: 1.6 log10 reduction) (Figure 3). This indicates that UV light may induce aggregation by decreasing cell viability and thereby rendering the cell membrane surface charge less negative. The UV-damaged cells attach more readily, creating aggregates within which the active cells are shielded from the UV light, decreasing UV efficiency.
On the contrary, the particle area of S. aureus strains was increasing or decreasing, regardless of UV treatment or whether aggregating or not, indicating that UV-induced aggregation also may be governed by species-specific characteristics. This observation regarding S. aureus also has been documented before by Lee et al. (2018).
Conclusions
- Aggregating strains were more UV tolerant among and within species, and UV efficiency increased when the aggregates of S. aureus were broken, indicating that aggregation may lead to light-shielding.
- A high microbial population reduction was observed at the lowest UV doses applied, followed by tailing effect in all the strains.
- The particle area increased significantly in the UV-treated samples for all the food processing water strains, whether aggregating or not, from the lowest UV dose applied, but there was no further significant increase at the higher doses.
- The significant increase in particle area and the high microbial reduction were observed at the lowest UV dose applied, indicating that UV may induce aggregation by reducing cell viability and thereby the negative cell membrane charge and the cell repulsion.
- UV did not increase the particle area in any of the S. aureus strains, indicating that UV-induced aggregation also may be species dependent.
Water reuse in the food processing industry is necessary to decrease the water footprint and contribute to sustainability. To widen water reuse applications in the food industry, more research is needed on UV efficiency for food processing water treatment to ensure the quality and safety of the water reuse practices. Focus should be given on the low % transmittance and high microbial population levels of the food processing water matrices, the UV tolerance of target microorganisms for each production, microbial survival mechanisms – such as aggregation – and modeling of UV inactivation considering the tailing phenomenon.
This study can be found in detail in the original article:
E. Vitzilaiou, A.M. Kuria, H. Siegumfeldt, M.A. Rasmussen, S. Knøchel, The impact of bacterial cell aggregation on UV inactivation kinetics, Water Res. 204 (2021) 117593. https://doi.org/10.1016/j.watres.2021.117593
Contact: Eirini Vitzilaiou, eirini@food.ku.dk
References
- S. Casani, S. Knøchel, Application of HACCP to water reuse in the food industry, Food Control. 13 (2002) 315–327. https://doi.org/10.1016/S0956-7135(02)00037-3.
- E. Vitzilaiou, I.M. Stoica, S. Knøchel, Microbial biofilm communities on Reverse Osmosis membranes in whey water processing before and after cleaning, J. Memb. Sci. 587 (2019). https://doi.org/10.1016/j.memsci.2019.117174.
- I.M. Stoica, E. Vitzilaiou, H. Lyng Røder, M. Burmølle, D. Thaysen, S. Knøchel, F. van den Berg, Biofouling on RO-membranes used for water recovery in the dairy industry, J. Water Process Eng. 24 (2018) 1–10. https://doi.org/10.1016/j.jwpe.2018.05.004.
- H. Chick, An Investigation of the Laws of Disinfection, J. Hyg. (Lond). 8 (1908) 92–158. https://doi.org/10.1017/s0022172400006987.
- H.E. Watson, A Note on the Variation of the Rate of Disinfection with Change in the Concentration of the Disinfectant, J. Hyg. (Lond). 8 (1908) 536–542. https://doi.org/10.1017/s0022172400015928.
- E.R. Blatchley, N. Dumoutier, T.N. Halaby, Y. Levi, J.M. Laîne, Bacterial responses to ultraviolet irradiation, Water Sci. Technol. 43 (2001) 179–186. https://doi.org/10.2166/wst.2001.0614.
- H. Mamane-Gravetz, K.G. Linden, Relationship between physiochemical properties, aggregation and u.v. inactivation of isolated indigenous spores in water, J. Appl. Microbiol. 98 (2005) 351–363. https://doi.org/10.1111/j.1365-2672.2004.02455.x.
- M.J. Mattle, T. Kohn, Inactivation and tailing during UV254 disinfection of viruses: Contributions of viral aggregation, light shielding within viral aggregates, and recombination, Environ. Sci. Technol. 46 (2012) 10022–10030. https://doi.org/10.1021/es302058v.
- K. Oguma, R. Kita, H. Sakai, M. Murakami, S. Takizawa, Application of UV light emitting diodes to batch and flow-through water disinfection systems, Desalination. 328 (2013) 24–30. https://doi.org/10.1016/j.desal.2013.08.014.
- S.E. Beck, H. Ryu, L.A. Boczek, J.L. Cashdollar, K.M. Jeanis, J.S. Rosenblum, O.R. Lawal, K.G. Linden, Evaluating UV-C LED disinfection performance and investigating potential dual-wavelength synergy, Water Res. 109 (2017) 207–216. https://doi.org/10.1016/j.watres.2016.11.024.
- G.-Q. Li, W.-L. Wang, Z.-Y. Huo, Y. Lu, H.-Y. Hu, Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli, Water Res. 126 (2017) 134–143. https://doi.org/10.1016/j.watres.2017.09.030.
- P.O. Nyangaresi, Y. Qin, G. Chen, B. Zhang, Y. Lu, L. Shen, Effects of single and combined UV-LEDs on inactivation and subsequent reactivation of E. coli in water disinfection, Water Res. 147 (2018) 331–341. https://doi.org/10.1016/j.watres.2018.10.014.
- D.C. Shoults, N.J. Ashbolt, Decreased efficacy of UV inactivation of Staphylococcus aureus after multiple exposure and growth cycles., Int. J. Hyg. Environ. Health. 222 (2019) 111–116. https://doi.org/10.1016/j.ijheh.2018.08.007.
- E. Vitzilaiou, S.D. Aunsbjerg, N.A. Mahyudin, S. Knøchel, Stress Tolerance of Yeasts Dominating Reverse Osmosis Membranes for Whey Water Treatment, Front. Microbiol. 11 (2020) 816. https://doi.org/10.3389/fmicb.2020.00816.
- M. Alhede, K.N. Kragh, K. Qvortrup, M. Allesen-Holm, M. van Gennip, L.D. Christensen, P.Ø. Jensen, A.K. Nielsen, M. Parsek, D. Wozniak, S. Molin, T. Tolker-Nielsen, N. Høiby, M. Givskov, T. Bjarnsholt, Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface attached biofilm, PLoS One. 6 (2011) e27943–e27943. https://doi.org/10.1371/journal.pone.0027943.
- K.N. Kragh, J.B. Hutchison, G. Melaugh, C. Rodesney, A.E.L. Roberts, Y. Irie, P.Ø. Jensen, S.P. Diggle, R.J. Allen, V. Gordon, T. Bjarnsholt, Role of Multicellular Aggregates in Biofilm Formation, MBio. 7 (2016) e00237-16. https://doi.org/10.1128/mBio.00237-16.
- J. Haaber, M.T. Cohn, D. Frees, T.J. Andersen, H. Ingmer, Planktonic aggregates of Staphylococcus aureus protect against common antibiotics, PLoS One. 7 (2012) e41075. https://doi.org/10.1371/journal.pone.0041075.
- M.J. Mattle, B. Crouzy, M. Brennecke, K. R. Wigginton, P. Perona, T. Kohn, Impact of Virus Aggregation on Inactivation by Peracetic Acid and Implications for Other Disinfectants, Environ. Sci. Technol. 45 (2011) 7710–7717. https://doi.org/10.1021/es201633s.
- H. Lee, Y. Jin, S. Hong, Understanding possible underlying mechanism in declining germicidal efficiency of UV-LED reactor, J. Photochem. Photobiol. B Biol. 185 (2018) 136–142. https://doi.org/10.1016/j.jphotobiol.2018.06.001.
- E. Chatzisymeon, Inactivation of bacteria in seafood processing water by means of UV treatment, J. Food Eng. 173 (2016) 1–7. https://doi.org/10.1016/j.jfoodeng.2015.10.027.
- K.A. Soni, A.K. Balasubramanian, A. Beskok, S.D. Pillai, Zeta Potential of Selected Bacteria in Drinking Water When Dead, Starved, or Exposed to Minimal and Rich Culture Media, Curr. Microbiol. 56 (2008) 93–97. https://doi.org/10.1007/s00284-007-9046-z.
- A. Ignat, L. Manzocco, I. Bartolomeoli, M. Maifreni, M.C. Nicoli, Minimization of water consumption in fresh-cut salad washing by UV-C light, Food Control. 50 (2015) 491–496. https://doi.org/10.1016/j.foodcont.2014.09.036.
- M.V. Selma, A. Allende, F. López-Gálvez, M.A. Conesa, M.I. Gil, Disinfection potential of ozone, ultraviolet-C and their combination in wash water for the fresh-cut vegetable industry, Food Microbiol. 25 (2008) 809–814. https://doi.org/https://doi.org/10.1016/j.fm.2008.04.005.
- W.A.M. Hijnen, E.F. Beerendonk, G.J. Medema, Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review, Water Res. 40 (2006) 3–22. https://doi.org/10.1016/j.watres.2005.10.030.
- K. Song, M. Mohseni, F. Taghipour, Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review, Water Res. 94 (2016) 341–349. https://doi.org/10.1016/j.watres.2016.03.003.
- K. Kollu, B. Örmeci, UV-induced self-aggregation of E. coli after low and medium pressure ultraviolet irradiation, J. Photochem. Photobiol. B Biol. 148 (2015) 310–321. https://doi.org/10.1016/j.jphotobiol.2015.04.013.