Oliver Lawal, CEO, AquiSense Technologies
Rich Simons, Ph.D., product development scientist, AquiSense Technologies
Anna Herman, applications engineer, AquiSense Technologies
Jennifer Pagan, Ph.D., CTO, AquiSense Technologies
Since its emergence in 2019 SARS-CoV-2, the virus behind the COVID-19 pandemic, has had a global impact. Solutions for controlling the virus are urgently needed to reduce its spread and UV light is known to inactivate all pathogens given a sufficient dose. The UV dose response of SARS-CoV-2 is needed to effectively apply this technology and fight the pandemic. As with other pathogens, this relationship can vary based on wavelength, UV lamp type, and the strain of the virus.
This article reviews the status of research into the dose response relationship, covering published data from numerous sources. The wide range of experimental procedures applied leads to substantial variability across the literature, though a convergence towards three key conclusions can be seen.
- tableIt is clear that UV-C radiation can reduce infectivity of SARS-CoV-2 to a high degree,
- Variation in susceptibility across the UV-C spectrum is relatively small (though more work is needed in this area),
- The D90 of SARS-CoV-2 (90% reduction) is very likely to be in the range 1 – 5 mJ/cm2.
The first documented infections from a novel virus, a member of the Coronaviridae family, occurred in Wuhan province, China, in December 2019. By January 2020, the infection was identified to be the result of a new virus, which was originally named 2019-nCov by the WHO and later renamed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as it will be referred to in the rest of this review. The virus can be spread through respiratory droplets in the air or via contact with infected surfaces.1 In an effort to reduce its spread, many have tried to interrupt transmission by inactivating the virus ex vivo.
Ultraviolet (UV) radiation is a common method of disinfection and is popular for its lack of chemicals and effectiveness against all known microorganisms. It can be used on surfaces, aerosolized particles or water, and is energy efficient. The UV spectrum spans wavelengths from 100 to 400 nm, but the UV-C range (200 to 280 nm) displays the strongest disinfecting properties.2 Many different sources, including low or medium-pressure mercury lamps (LP and MP) and light emitting diodes (LEDs) can be used to produce UV-C light.
To quantify the inactivation properties of UV light, a dose response relationship is needed. The inactivation is commonly presented as a log reduction value (LRV):

Where is the starting concentration of a pathogen and is the concentration of the pathogen after exposure; common units in viral studies are PFU/mL or TCID50/mL. In surface exposure studies, UV dose [mJ/cm2] is the product of UV irradiance across the exposed sample [mW/cm2] and the exposure time [s]; where the target pathogen is suspended in a fluid (air, water, etc.) UV dose (fluence) definitions become more complex.
This review summarizes work that has been completed, or that is in progress, to study the UV dose response relationship of SARS-CoV-2. First, completed research is summarized and categorized based on the wavelength and UV source. Ongoing studies are then highlighted to display which research areas are still in progress. Finally, gaps in current research are identified and future research goals are discussed.
Published research
This section outlines studies that have been completed on the dose response relationship for SARS-CoV-2 and UV light. These studies have taken place globally and cover a variety of wavelengths, types of samples, culture methodologies and UV sources. Not all studies gave complete UV exposure methodologies including wavelength or actual dose. The studies included in this review are outlined in Table 1.
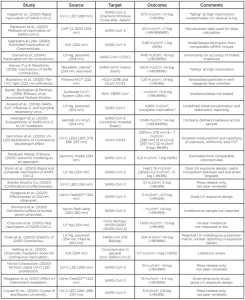
Inagaki, H., et al. (2020) [https://doi.org/10.1080/22221751.2020.1796529]
SARS-CoV-2 isolated from the Diamond Princess cruise ship (Japan) was exposed to a UV-C LED source, nominal 280 nm peak emission. The virus was propagated in Vero cells for 48 hours, and the supernatants separated out and used for testing. The virus was mixed with saline solution to 2.0×104 PFU/mL and exposed to 3.75 mW/cm2 for 1, 10, 20, 30, and 60s with 3 trials each. A 3.3-log inactivation (limit of detection) was reached at between 10 and 20 s exposure (37.5 to 75 mJ/cm2); higher resolution of the study in the 10 to 50 mJ cm-2 UV dose range would have improved the quality of these results.
Patterson, E., et al. (2020) [https://doi.org/10.1093/infdis/jiaa507]
Various physical and chemical methods for inactivation of SARS-CoV-2 including heat, detergents and UV were assessed. Samples contained 1.145×107 PFU/mL initially. UV inactivation was achieved using a UVP CL-1000 Shortwave Crosslinker cabinet (254 nm), with plates exposed to UV doses of 10 to 800 mJ/cm2 with 3 trials each. Complete inactivation (>7-log inactivation) was reached at 40 mJ/cm2. Sufficient datapoint in the 0 to 40 mJ/cm2 region show a clear log-linear trend, though direct measurements of UV irradiance at the sample surface were not made and the true UV dose may differ from that reported.
Sagripanti, J., & Lytle, C. (2020) [https://doi.org/10.1111/php.13293]
Inactivation of SARS-CoV-2 was estimated using a solar inactivation model based on the UV sensitivity of other ssRNA viruses such as influenza A. The model predicted the UV-C dose for a 1-log (90%) reduction at 0.7 mJ/cm2, and 2-log (99%) reduction at 2.8 mJ/cm2. These results may be indicative of scale, though no experimental data were presented.
Darnell, M., et al. (2004) [https://doi.org/10.1016/j.jviromet.2004.06.006]
SARS-CoV was exposed to UV-A and UV-C light sources, gamma irradiation, heat treatment, formaldehyde and glutaraldehyde treatment, pH treatment or detergent. A low-pressure mercury UV-C light source (254 nm) was placed above a 24-well plate, providing a measured irradiance of 4.016 mW/cm2 at 2 cm distance. Samples with an initial concentration of 106.33 TCID50/mL were exposed to UV radiation for 1 to 15 minutes, with >5-log inactivation observed after 6 minutes (1,445 mJ/cm2). Though a measured irradiance is provided, the specification of 2 cm displacement to a multi-well plate indicate that the sample is not likely to have received a uniform exposure; further the sensor is noted to have been used for measurement of UV-C (254 nm) and UV-A (365 nm) radiation, without discussion of appropriate recalibration. These limitations in methodology and reporting suggest that the results may be unreliable.
Kariwa, H., Fujii, N., Takashima, I., (2006) [https://doi.org/10.1159/000089211]
The Hanoi strain of SARS-CoV was treated with Povidone-iodine products, chemical reagents, heat treatment or UV radiation. A UV light source (assumed to be a UVGI bulb emitting at 254 nm) delivered a measured irradiance of 134 W/cm2 to viral suspension at 3.8×107 TCID50/mL. Irradiation for 15 minutes (121 mJ/cm2) resulted in >5-log reduction in viral titer, though these data also showed significant ‘tailing’ effects, where further inactivation becomes increasingly difficult. Whereas the standardized collimated beam protocol and correction factors were not applied, the methodology and reporting indicate that it is a reliable study.
Buonanno, M., et al. (2020) [https://doi.org/10.1038/s41598-020-67211-2]
This study quantified the inactivation capacity of far UVC light (222 nm) on HCoV-229E and HCoV-OC43. Aerosol samples were irradiated in a single-pass flow chamber. The flow rate through the system was 12.5 L/min and the chamber volume was 4.2 L, giving an ideal residence time of 20s. Three KrCl* excimer lamps provided an average fluence rate of 100 W/cm2 through the chamber. Sheets of plastic were used to decrease transmission and adjust dose rather than altering the lamp position/power. Dose-survival data was used to determine D90 values and it was estimated that 1.7 and 1.2 mJ/cm2 were needed for 3 log removal of HCoV-229E and HCoV-OC43, respectively. Accurate quantification of dose delivery to an aerosolized target is challenging, though the equipment and methodology presented are of high quality. Differences in droplet transit times through the chamber will result in some distribution in dose delivery, though the use of bulk flow rates and inactivation are appropriate.
Bedell, K., Buchaklian, A.H., Perlman, S. (2016) [https://dx.doi.org/10.1017/ice.2015.348]
MHV-A59 and MERS-CoV were treated with a Sufacide UV-C system (a commercial surface disinfection device employing three 254 nm UV bulbs). Initial concentrations of 1.6×108 PFU/mL MVH-A59 and 8.9×105 PFU/mL MERS-CoV were exposed to the UV device for 0 to 60 minutes, though no irradiance or UV dose values were reported. An undetectable level or 6.11-log removal of MHV-A59 was reached in 10 minutes, and an undetectable level of 5.9-log removal of MERS-CoV was reached in five minutes. The lack of reported UV dose values limits transferability of these data, though they may be anecdotally considered to show that high levels of inactivation may be achieved using existing UV systems and processes.
Ansaldi, F., et al. (2004) [https://bit.ly/3pTICh8]
The response of SARS-CoV, Influenza, and Respiratory Sincytial Virus (RSV). to sodium hypochlorite, ethanol, benzalkonium-chloride, chlorohexidine digluconate, 1-benzil-chlorophenol, peracetic acid or ultraviolet radiation was investigated. Viral samples were applied to a “20 cm2 plate” and exposed to an irradiance 40 mW/cm2 of 254 nm UV-C for up to 30 minutes; no further details are provided about the plate, its orientation relative to the source, the deposition method, or the uniformity of irradiation. Detection by PCR indirect immune fluorescence and infectivity assay showed complete inhibition after 1 minute (2,400 mJ/cm2), though the initial concentration is not defined. Limitations in the reporting and use of atypical methodologies limit the transferability of these findings.
Heilingloh, C., et al. (2020) [https://dx.doi.org/10.1016/j.ajic.2020.07.031]
SARS-CoV-2 isolated from a patient in University Hospital Essen was exposed to UV-A and/or UV-C radiation to determine its susceptibility. An initial titer of 5×106 TICD50/mL was reduced below the limit of detection (<100 TCID50/mL) under 1048 mJ/cm2 of UV-C radiation at 254 nm; ~1-log inactivation was demonstrated under UV-A (broadband) irradiation up to 500 mJ/cm2. A UV-C irradiance of 1,940 μW/cm2 was measured at a 3 cm displacement from the UV-C source, though uniformity across the multi-well plate was not reported. No further corrections between measured irradiance and actual fluence rate are reported.
Gerchman, Y., et al. (2020) [https://dx.doi.org/10.1016/j.jphotobiol.2020.112044]
The coronavirus HCoV-OC43 was exposed to radiation from various LEDs across the UV-C and UV-B spectrum. LEDs with peak wavelengths of 267, 279, 286, and 297 nm produced irradiances of 0.16, 0.43, 0.39, and 0.58 mW/cm2 respectively. Custom exposure equipment was used to expose samples within a multi-well plate and the irradiance was mapped across the sample surface; transmissivity of the viral suspension was measured across the UV spectrum (>98%), and experimental design limited errors in the translation of measured irradiance to true fluence rate. Corrections for surface reflection of the sample were not made and so the reported fluence may be ~2.5% above the true values, though this does not affect the conclusions of this work. An initial concentration of 8×105 PFU/mL resulted in a limit of quantification up to 3-log reduction; beyond this, substantial ‘tailing’ was observed. Exposure to shorter LED wavelengths, 267 and 279 nm, produced 3-log inactivation at a fluence of 6-7 mJ/cm2, while longer LED wavelengths, 286 and 297 nm, required at a dose of 13 and 32 mJ/cm2 respectively.
Pendyala, B., et al. (2020) [https://doi.org/10.3389/fmicb.2020.572331]
A genomic model was used to estimate the susceptibility of SARS-CoV-2 to disinfection by UV-C radiation. The model was based on pyrimidine dinucleotide frequency and used data from other ssRNA viruses such as MS2, Murine norovirus, and SARS-CoV-1. The 254 nm UV-C D90, (dose for 1-log inactivation) of SARS-CoV-2 and MERS-CoV were predicted to be 2.1 and 2.8 mJ/cm2, respectively, showing coronaviruses of this type to be highly susceptible to UV-C radiation compared to other viruses.
Storm, N., et al. (2020) [https://doi.org/10.1038/s41598-020-79600-8]
A sponsored study by Signify Research investigated the effect of 254 nm UV-C on SARS-CoV-2. A suspension of SARS-CoV-2, matrix not specified, was applied to a plastic tissue culture dish, and exposed within a custom-designed UV-C chamber either whilst wet, or after a 2-hour drying step. Measurements within the chamber mapped the irradiance distribution and confirmed an irradiance 0.849 mW/cm2 across the sample surface; the true fluence across a surface-bound 5 μL droplet is poorly defined, though the dried samples provide a reasonable facsimile of the intended use case for this technology. Initial concentrations of 7.33×103 PFU/mL resulted in recoverable concentrations ~2×103 for wet samples, and 5×102 for dried samples; this resulted in a difference in range of outcomes between the two studies and the need for caution in interpretation of the linear-scale ‘remaining infectivity’ figures as presented. >3-log inactivation was achieved in the ‘wet’ condition for a UV exposure of ~7.6 mJ/cm2, and ~3-log inactivation was achieved in the ‘dry’ condition for a UV exposure of ~4.2 mJ/cm2.
Stanley Electric Co., Ltd. (2020) [https://bit.ly/3txNdb3]
A press release by Stanley Electric Co., Ltd. claimed >99.9% inactivation of SARS-CoV-2. A 10 mL sample at an initial concentration of 5×105 PFU/mL was exposed to a nominal 265 nm UV-C LED source up to 5.1 mJ/cm2. No further experimental data are reported, and no peer-reviewed paper has yet been published.
Kitagawa, H., et al. (2020) [https://doi.org/10.1016/j.ajic.2020.08.022]
An Ushio Care222™ UV-C device was used to inactivate SARS-CoV-2. The UV-C source incorporates a KrCl* excimer lamp and optical filter, producing a narrow emission peak about 222 nm; radiometric measurements at 24 cm confirmed an irradiance of 0.1 mW/cm2. Thin layers of viral suspension (initial concentration 5×106 TCID50/mL) were allowed to dry before exposures of 1 to 30 mJ/cm2. An exposure of 3 mJ/cm2 resulted in ~2.5-log inactivation (limit of detection) when assessed by TCID methodology; the copy number of SARS-CoV-2, analyzed by RT-qPCR, did not change under UV exposure up to 30 mJ/cm2.
Simmons, S., et al. (2020) [https://dx.doi.org/10.1017/ice.2020.399]
A pulsed xenon ultraviolet disinfection system (Xenex PXUV4D) was used to inactivate SARS-CoV-2 on hard and soft surfaces (chamber slides and N95 respirator material). 20 μL droplets of viral suspension, initial titer of 1.3×107 PFU/mL, were applied to the test surfaces, spread, and allowed to dry for 55 minutes. Samples were then exposed to the pulsed UV source for 1, 2, or 5 minutes at 1 m displacement; though the experimental condition suggests uniform exposure of the samples, this was not reported, nor were irradiance or total exposure measurements. >4-log inactivation was achieved across both sample materials, though inactivation was more readily achieved on the hard surface sample than the soft, porous N95 material. The lack of detail reported on the exposure conditions limits transferability of these data, though the study clearly demonstrates the ability of UV to achieve substantial inactivation of SARS-CoV-2, and the impact of surface texture to such applications.
Criscuolo, E., et al. (2020) [https://doi.org/10.1080/22221751.2021.1872354]
Inactivation effects of UV-C and ozone on SARS-CoV-2 were tested across different surface substrates. Glass, plastic, gauze, wood, fleece, and wool substrates were inoculated with a SARS-CoV-2 suspension of initial concentration 8.2×105 PFU/mL, and subsequently exposed to UV radiation or ozone. The UV apparatus appears to be a facsimile of a previously reported irradiation chamber design (doi: 10.9745/GHSP-D-20-00218) and irradiance values (1.8 mW/cm2) are reported as approximate. No discussion of irradiance uniformity is reported, and the use of a 24-well plate indicates that the reported UV exposure may not be accurate. Inactivation generally correlated to surface porosity/roughness, though uncertainty the true UV irradiance delivered limits the utility of inactivation data vs absolute UV exposure.
Duan, S., et al. (2003) [https://bit.ly/3tyCjBZ]
SARS-CoV (P9 strain) was exposed to heat or UV irradiation. Samples containing 106 TCID50/mL, suspended in natural culture medium, were irradiated by an (assumed) 254 nm source to >90 W/cm2 for 15 to 150 minutes. The exposure was conducted on samples within a 96-well plate at 80 cm displacement; though uniform irradiance may be expected, UVT was not reported, and the high NOM content of the media may impact the accuracy of fluence values as presented. Viral infectivity was substantially reduced by >81 mJ/cm2 and reduced to an undetectable level following irradiation to >324 mJ/cm2.
Heßling, M., et al. (2020) [https://dx.doi.org/10.3205/dgkh000343]
A review of published UV dose response data for SARS-CoV-2 and other coronaviruses was made. Most data related to 254 nm (LP Hg) sources and were presented in the form of D90 values (UV dose per log inactivation). It was summarized that coronaviruses in general (including SARS-CoV-2) were susceptible to UV disinfection, and that a most-probable median D90 was 3.7 mJ/cm2 (upper limit 10.6 mJ/cm2).
Nichia Co. (2021) [http://www.nichia.co.jp/en/about_nichia/2020/2020_121701.html]
Press release summarizing bench-top study of a SARS-CoV-2 disinfection by a commercially available handheld UV-C LED wand. No detail is provided on the test protocol, suspension media, initial concentration, sample dimensions, or enumeration methodology; an embedded photograph shows a linear array of UV-C LEDs displaced 50 mm above a multi-well plate, with a stated irradiance of 1.7 mW/cm2 though no discussion of uniformity is reported. A roughly linear dose response curve is presented through to >99.99% inactivation at a UV exposure of 51 mJ/cm2. No comment is made on forthcoming detailed reporting or peer reviewed publication of this work.
Kitagawa, H., et al. (2021) [https://doi.org/10.1016/j.pdpdt.2021.102184]
Investigation of irradiance level and continuous/intermittent exposure effects on overall inactivation of SARS-CoV-2 by 222 nm radiation. No significant differences were observed between experiments of varying irradiance or intermittent/continuous exposure, supporting the Bunsen-Roscoe dose reciprocity model (total exposure governs inactivation, rather than rate of exposure). A Care222™ UV source was positioned 24 cm from the viral samples, giving uniform exposure. Exposure up to 3 mJ/cm2 resulted in >3-log inactivation in all exposure conditions, with substantial ‘tailing’ at higher exposures, up to ~4.5-log inactivation at 15 mJ/cm2.
Crystal IS Inc. (2020) [https://bit.ly/36OuhLo]
Crystal IS reported in a press release the use of LEDs at peak wavelengths between 260 to 270 nm to inactivate SARS-CoV-2. The 268 nm UV-C LED source is stated to deliver an irradiance of 1.25 mJ/cm2, though no detail is provided on the test protocol, suspension media, initial concentration, sample dimensions, or enumeration methodology. UV-C LED sources at 260 nm and 270 nm peak emission are also reported to deliver a 5 mJ/cm2 UV exposure, though no irradiance is reported. A maximum 2.8-log inactivation was achieved from the 5 mJ/cm2 exposure to the 268 nm and 270 nm source, whereas a slightly lower 2.6-log inactivation was achieved from the 260 nm source. The press release goes on to contrast these findings against the work of Inagaki et al. using 280 nm UV-C LEDs (summarized above), though insufficient detail is provided in this release to comment on the validity of such a comparison. No comment is made on forthcoming detailed reporting or peer reviewed publication of this work.
Current Research
Table 2 summarizes publications currently in pre-print but that have not yet passed peer-review. Many other studies are likely occurring, but their data is not yet publicly available.

Future research goals and conclusion
 Numerous studies have been completed on SARS-CoV-2 and similar coronaviruses; however, variations in experimental set ups and UV wavelengths, and incomplete dose data leave researchers without a clear and consistent dose response relationship. Studies reporting exposure time only and not irradiance or intensity limit the ability to derive true UV dose values.
Numerous studies have been completed on SARS-CoV-2 and similar coronaviruses; however, variations in experimental set ups and UV wavelengths, and incomplete dose data leave researchers without a clear and consistent dose response relationship. Studies reporting exposure time only and not irradiance or intensity limit the ability to derive true UV dose values.
Low-pressure mercury vapor and KrCl* excimer lamps are the most commonly studied UV sources, though other UV sources such as UV-C LEDs and pulsed UV are also presented. Between high-quality studies, there appears to be good agreement of UV dose response values across the UV-C spectrum, indicating that wavelength does not play a substantial role in disinfection efficacy. However, further work is required to refine this understanding build transferability of inactivation studies across the UV-C spectrum.
Most importantly, future tests must be vigilant in recording the methods used and the actual dose applied, not simply exposure time so that the tests could be replicated. While there is much work left to be done, the UV community is on the right track to gather the needed data and work together to stop the spread of SARS-CoV-2.
Acknowledgements
The authors wish to acknowledge the support of all named researchers for generously providing details of their important work.
References
- Liu, Y.C., Kuo, R.L., Shih, S.R., (2020) COVID-19: The first documented coronavirus pandemic in history, Biomedical Journal, 43, 4, pp 328 – 333. doi: 10.1016/j.bj.2020.04.007
- IESNA, 2000b. IESNA Lighting Handbook, 9th Edition. ed. Rea M.S. Illuminating Engineering Society of North America, New York, 2000