Mahdiyeh Hasani, CPHAZ, Department of Food Science, University of Guelph, Ontario
Dr. Tatiana Koutchma, Agriculture and AgriFood Canada, Guelph, Ontario
Corresponding author Dr. Keith Warriner, CPHAZ, Department of Food Science, University of Guelph, Ontario
As scientists and companies rush to find a vaccination for SARS-CoV-2, there is an emerging field of modulating plant defenses to protect against plant pathogens that lead to crop failure or premature spoilage. Traditionally, plant protection during preharvest is via applying pesticides and to keep pathogens at bay. At postharvest, attempts are made to reduce the spoilage microflora via washing coupled with maintaining optimal storage conditions (Ali et al. 2018).
Yet, plants have developed defense systems to protect against invasion by would-be pathogens that could be exploited to extend the shelf life in a natural way. Several biological, chemical and physical approaches to modulating plant-defense mechanisms fall under the term hormesis, which is derived from Greek for “to set in motion.” Essentially, hormesis is applied to describe a process that, when administered at a low dose, will elicit a protective effect – that is, what doesn’t kill you makes you stronger.
The following will provide an overview of current progress of UV-based methods to modulate plant defenses to provide protection against microbial and autolytic processes. The approach offers a way of reducing reliance on pesticides to control plant pathogens and extend shelf life, thereby positively affecting food waste.
Plant defenses against pathogens and stress
Vaccines were among the early breakthroughs in modern science and key to controlling deadly diseases, such as smallpox and measles. Vaccines essentially work by priming the immune system to react rapidly when the body comes under attack from the target infectious agent. Like humans, plants have had to evolve defense systems to protect against pathogens, insects and environmental stresses (sun, heat, draught). Obviously, the defense systems in plants are very different than those of humans, although in some ways they are similar in terms of different levels of activation. Specifically, plants have an innate defense system that is constitutive and includes physical barriers (for example, cell walls, waxy coating), in addition to antimicrobials, such as essential oils.
The second layer of defense is the localized induced resistance (LIR) that functions to defend against opportunistic microbes by producing antimicrobial compounds but with no necrosis (plant tissue damage).
If faced with an invading pathogen, the hypersensitive response (HR) is induced, which releases an oxidative burst that results in localized necrosis to contain the spread of the pathogen. At the same time, the plant hormones (salicylic acid, jasmonic acid and ethylene) circulate around the plant via the vascular system to activate HR genes that are primed by the systemic acquired resistance (SAR).
Similar to when humans receive a vaccine, the SAR primes the plant HR to rapidly respond to plant pathogens. With regards to UV-induction, the goal is to activate the LIR and SAR to provide protection against pathogens and insects, thereby preventing the spread of infection without leading to plant programmed death at preharvest or spoilage at postharvest (Zhang and Jiang 2019).
Preharvest application of UV-B and UV-C
UV-B induction of plant defenses
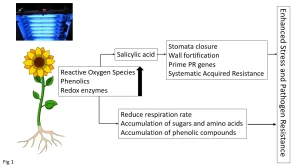
The majority of work to date on UV-induced plant defenses has been with UV-B (280 to 315 nm) that applies doses over a period of days (Ballare et al. 2011). The effect of UV-B on plant defenses remains an active area of research, with many aspects still to be elucidated. Currently, it is thought that upon the initial exposure of plants to UV-B there is a radical oxygen species (ROS) burst that then stimulates the synthesis of redox enzymes (catalase, glutathione reductase, super-oxidase-dismutase) and phenolic (antioxidant) compounds (Yadav et al.) (Figure 1).
With regards to the latter, the types and concentration of phenolic acids change over the course of exposure. For example, hydroxycinnamic acid is among the early phenolics formed that provide direct protection by absorbing UV photons and acting as an antioxidant. Other non-flavonoids develop over prolonged exposure to UV-B and are plant specific. For example, gallic acid and caffeic acid accumulated in grapes within a seven-day exposure to UV-B. More significant is the synthesis of flavonoids, such as anthocyanins, that act as antioxidants, chelating agents, flavor compounds and antimicrobials (Barrera et al. 2020).
The free-radical burst also stimulates synthesis of the plant hormone salicylic acid that activates one of the main branches of the plant defense system (Bravo et al. 2019). Specifically, salicylic acid results in the thickening of plant cell walls and closure of stomata, thereby increasing the physical protection against pathogen invasion. The cell wall degrading enzymes are inhibited, thereby retarding softening, and the PR genes become primed as part of the SAR. Collectively, the induction of redox enzymes, accumulation of phenolics and activation of SAR collectively enhance the stress tolerance of plants, along with resistance to plant pathogens (Figure 1). However, overexposure to UV-B results in senescence through overactivation of SAR, in addition to activation of the jasmonic acid regulatory pathways, leading to growth inhibition and the ultimately programmed cell-death.
UV-C induction of plant defenses
UV-C (200 to 280 nm) can directly inactivate microbes through reacting with nucleic acids that cause lethal mutations and proteins that affect cell function. Therefore, plant pathogens can be directly inactivated by UV-C, although it should be noted nucleic acids of plants also can be affected, leading to negative effects on plant health.
The effect of UV-C on inducing plant defenses remains to be elucidated, and there is debate if it follows UV-B or is distinct (Zhang and Jiang 2019). It has been reported that the same ROS burst, along with up regulation of redox enzymes and induction of SAR, occurs with UV-C exposure as with UV-B (Arrouft and Urban, 2020).
However, unlike UV-B, the duration of UV-C is measured in seconds rather than days. From studies performed to date, the UV-C intensity is more critical than the total dose, with 1 kJ/m2 being effective. In addition, plants also need to be held for a four-hour dark period following the UV-C exposure (Janisiewicz et al. 2016).
By applying the UV-C treatment to tomatoes or lettuce inoculated with Botrytis cinereal, it was possible to reduce lesion formation by the plant pathogens by 35% and 17% respectively (Arrouft and Urban, 2020). In a further example, strawberry plants treated once a week with 60 s exposure to UV-C (21 mW/m2) could effectively eliminate mildew caused by Podosphera aphanis (Janisiewicz et al. 2016).
In addition to activating plant defenses, it also is likely that UV-C impacts on the physiology of mildew-causing molds. Indeed, there is evidence that UV-C inhibits mold spore germination, thereby effectively making the microbe nonviable (Zhu et al. 2019). It is theorized that the four-hour holding period following UV-C exposure prevents photo-repair of damaged DNA, although the mechanism remains unclear (Takeda et al. 2019). One can envision that future research likely will discover the mildew-protecting effect is a combination of UV-C mediated physiological changes in plants and mildew causing mold.
UV-C treatment of crops (strawberries, tomatoes, basil, lettuce) to control mildew is being researched in Europe, Florida and California, with a view of eliminating, or at least reducing, the use of chemical fungicides. Similar to UV-B, overexposure of plants to UV-C results in detrimental effects, with doses >230 J/m2 or excessive treatment repetitions, resulting in damage, delayed flowering and stunted growth. The overdosing effect of UV-C is more critical compared to UV-B, although it is likely to depend on plant type and physiological condition.
Postharvest UV-B and UV-C treatment
There have been relatively few studies of the effect of UV-B on postharvest of fresh produce, probably due to the long exposure times required. However, studies have been performed with UV-C, both in terms of microbial inactivation and effect on plant physiology.
With regard to the latter, it has been demonstrated that spinach plant exposure to UV-C (1.5 kJ/m2) immediately before harvest increased the antioxidant content and shelf life at postharvest (Martinez-Sanchez et al. 2019). In a similar manner, preharvest apples exposed to UV-C had enhanced malic acid dehydrogenase activity and reduced concentration of malate (sharp apple flavor) during storage (Onik et al. 2019).
UV light has been shown to increase the rate of ripening in banana, tomato and citrus fruit (Hu et al. 2019). The increase in sugars and reduction in acidity is the result of upregulation of producing enzymes. Interestingly, in citrus fruit, UV-A increases the fructose content, UV-B the sucrose and UV-C glucose (Hu et al. 2019). This may suggest that the sugar composition (perceived sweetness) could be modulated by using the different doses at the appropriate UV wavelength.
Exposure of fruit to UV-C doses of 3.7 kJ/m2 is reported to stimulate antimicrobial compounds in preventing infection by plant pathogens. For example, anthocyanins in cherries, rishitin in tomatoes, scoparone in citrus and 6-methoxymellein in carrots accumulate with UV-C exposure that enhances control of spoilage microbes and can modify the flavor profile (Zhang and Jiang 2019) (Kokalj et al. 2019) (Gonzalez-Villagra et al. 2020). Tomatoes treated with UV-C light increase in antioxidant content during storage (Pataro et al. 2015).
Enzymes such as polyphenol oxidase, super-oxidase dismutase, peroxidase, catalase and phenylalanine ammonialyase also are stimulated by UV-C that can delay spoilage by Botrytis and protect against oxidative damage of fruit (Jin et al. 2017). In addition, as with preharvest UV-C treatment, postharvest exposure to germicidal-UV results in cell wall thickening while reducing the activity of degrading enzymes (pectin methylesterase and polygalacturonase) thereby making fruit more robust and reducing moisture loss (Prasanna et al. 2007; Charles et al. 2008).
Effect of gas phase-advanced oxidation based treatments on fruit shelf-life
Gas-phase advanced oxidation process (gAOP) is a UV-C-based method that generates hydroxyl-radicals from the photo-degradation of hydrogen peroxide and/or ozone. The process involves passing the produce to be decontaminated through a gAOP reactor where a hydrogen peroxide mist and ozone are introduced while simultaneously being illuminated with UV-C (Hasani and Warriner, 2019). The gas-phase AOP treatment has been demonstrated to directly inactivate human and plant pathogens on apples, lemons and other fresh produce (Murray et al. 2018; Hasani et al. 2019).

Although no studies have been undertaken on the effect of gAOP on plant physiology, there have been observations of shelf life extension of fruit that go beyond microbial reduction.
For example, strawberries treated with gAOP retained quality over a 15-day storage period (Figure 2). A similar effect was seen for other soft fruit (blackberries, blueberries and raspberries) treated with gAOP then stored at 4°C for 16 days.

Lemons inoculated with Penicillium digitiam using a probe applicator that drove spores of the mold 2 mm into the fruit peel were treated with gAOP, then held at room temperature for 10 days. With controls receiving no gAOP treatment, there was confluent growth of mold in contrast to those treated with gAOP, which exhibited no or minor growth (Figure 3).
In a further example, avocado (n=18) treated with gAOP retained quality over 10 days in storage. In comparison, in untreated controls, only three out of the 18 fruit remained acceptable at the end of the storage period (Figure 4).

It was interesting to note that the main symptom of spoilage of avocado was softening and discoloration of the inner flesh. Given that gAOP is a surface treatment, it is likely that the physiology of the avocado had been changed.
A similar effect has been observed with soft fruit treated with gas plasma, whereby the generation of free-radicals stimulates the synthesis of anti-oxidant compounds that provides protection during post-treatment storage (Pan et al. 2019).
Future prospective
The enhanced plant protection provided by UV-B, UV-C or gAOP exposure represents a new field of research that offers the promise of reducing the need for pesticides, in addition to shelf life extension. Such initiatives can reduce the risk of pesticide carriage on fresh produce and potentially toxic chemicals becoming disseminated in the environment. The extension of shelf life not only brings economic benefits but also reduces food waste.
Given the research area is new, there are key knowledge gaps on the type of UV, time of application and fate of key biomarkers (enzymes, metabolites) during postharvest storage. This is especially the case, given that the effect of treatment depends on the produce type, stage of plant development, UV wavelength and duration.
The complexity of plant defense regulation will likely mean there are additional factors, making for a complex process. Yet, if a “plant vaccine” can be developed it will provide yet another application of UV technology beyond microbial reduction.
Contact: Mahdiyeh Hasani, mahdiyehasani@gmail.com; Dr. Tatiana Koutchma, tatiana.koutchma@canada.ca; Dr. Keith Warriner, kwarrine@uoguelph.ca
References
- Aarrouf, J and Urban, L. (2020). Flashes of UV-C light: An innovative method for stimulating plant defenses. PLOSOne 15(7): e0235918. https://doi.org/10.1371/journal.pone.0235918
- Ali, A., Yeoh, W.K., Forney, C. and Siddiqui, M.W. (2018) Advances in postharvest technologies to extend the storage life of minimally processed fruits and vegetables. Critical Reviews in Food Science and Nutrition 58, 2632-2649.
- Ballare, C.L., Caldwell, M.M., Flint, S.D., Robinson, A. and Bornman, J.F. (2011) Effects of solar ultraviolet radiation on terrestrial ecosystems. Patterns, mechanisms, and interactions with climate change. Photochemical & Photobiological Sciences 10, 226-241.
- Barrera, A., Hereme, R., Ruiz-Lara, S., Larrondo, L.F., Gundel, P.E., Pollmann, S., Molina-Montenegro, M.A. and Ramos, P. (2020) Fungal Endophytes Enhance the Photoprotective Mechanisms and Photochemical Efficiency in the Antarctic Colobanthus quitensis (Kunth) Bartl. Exposed to UV-B Radiation. Frontiers in Ecology and Evolution 8, 13.
- Bravo, R.E., Chen, G., Grosser, K., Van Dam, N.M., Leiss, K.A. and Klinkhamer, P.G.L. (2019) Ultraviolet radiation enhances salicylic acid-mediated defense signaling and resistance to Pseudomonas syringae DC3000 in a jasmonic acid-deficient tomato mutant. Plant Signaling & Behavior 14, 7.
- Charles, M.T., Benhamou, N. and Arul, J. (2008) Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit – III. Ultrastructural modifications and their impact on fungal colonization. Postharvest Biology and Technology 47, 27-40.
- Gonzalez-Villagra, J., Reyes-Diaz, M., Alberdi, M., Mora, M.L., Ulloa-Inostroza, M. and Ribera-Fonseca, A.E. (2020) Impact of Cold-Storage and UV-C Irradiation Postharvest Treatments on Quality and Antioxidant Properties of Fruits from Blueberry Cultivars Grown in Southern Chile. Journal of Soil Science and Plant Nutrition, 8. 10.1007/s42729-020-00247-5
- Hasani, M and Warriner, K (2019). AOP for surface disinfection of fresh produce: From concept to commercial reality. UV Solutions Available at https://uvsolutionsmag.com/articles/2019/aop-for-surface-disinfection-of-fresh-produce-from-concept-to-commercial-reality/
- Hasani, M., Chudyk, J., Murray, K., Lim, L.T., Lubitz, D. and Warriner, K. (2019) Inactivation of Salmonella, Listeria monocytogenes, Aspergillus and Penicillium on lemons using advanced oxidation process optimized through response surface methodology. Innovative Food Science & Emerging Technologies 54, 182-191.
- Hu, L.P., Yang, C., Zhang, L.N., Feng, J. and Xi, W.P. (2019) Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 24, 15.
- Janisiewicz, W.J., Takeda, F., Nichols, B., Glenn, D.M., Jurick, W.M. and Camp, M.J. (2016) Use of low-dose UV-C irradiation to control powdery mildew caused by Podosphaera aphanis on strawberry plants. Canadian Journal of Plant Pathology 38, 430-439.
- Jin, P., Wang, H.Y., Zhang, Y., Huang, Y.P., Wang, L. and Zheng, Y.H. (2017) UV-C enhances resistance against gray mold decay caused by Botrytis cinerea in strawberry fruit. Scientia Horticulturae 225, 106-111.
- Kokalj, D., Zlatic, E., Cigic, B. and Vidrih, R. (2019) Postharvest light-emitting diode irradiation of sweet cherries (Prunus avium L.) promotes accumulation of anthocyanins. Postharvest Biology and Technology 148, 192-199.
- Martinez-Sanchez, A., Lozano-Pastor, P., Artes-Hernandez, F., Artes, F. and Aguayo, E. (2019) Preharvest UV-C treatment improves the quality of spinach primary production and postharvest storage. Postharvest Biology and Technology 155, 130-139.
- Murray, K., Moyer, P., Wu, F., Goyette, J.B. and Warriner, K. (2018) Inactivation of Listeria monocytogenes on and within Apples Destined for Caramel Apple Production by Using Sequential Forced Air Ozone Gas Followed by a Continuous Advanced Oxidative Process Treatment. Journal of food protection 81, 357-364.
- Onik, J.C., Xie, Y.J., Duan, Y.Q., Hu, X.J., Wang, Z.D. and Lin, Q. (2019) UV-C treatment promotes quality of early ripening apple fruit by regulating malate metabolizing genes during postharvest storage. Plos One 14, 17.
- Pan, Y.Y., Cheng, J.H. and Sun, D.W. (2019) Cold Plasma-Mediated Treatments for Shelf Life Extension of Fresh Produce: A Review of Recent Research Developments. Comprehensive Reviews in Food Science and Food Safety 18, 1312-1326.
- Pataro, G., Sinik, M., Capitoli, M.M., Donsi, G. and Ferrari, G. (2015) The influence of postharvest UV-C and pulsed light treatments on quality and antioxidant properties of tomato fruits during storage. Innovative Food Science & Emerging Technologies 30, 103-111.
- Prasanna, V., Prabha, T.N. and Tharanathan, R.N. (2007) Fruit ripening phenomena – An overview. Critical Reviews in Food Science and Nutrition 47, 1-19.
- Takeda, F., Janisiewicz, W.J., Smith, B.J. and Nichols, B. (2019) A new approach for strawberry disease control. European Journal of Horticultural Science 84, 3-13.
- Yadav, A., Singh, D., Lingwan, M., Yadukrishnan, P., Masakapalli, S.K. and Datta, S. Light signaling and UV-B-mediated plant growth regulation. Journal of Integrative Plant Biology, 23.
- Zhang, W.L. and Jiang, W.B. (2019) UV treatment improved the quality of postharvest fruits and vegetables by inducing resistance. Trends in Food Science & Technology 92, 71-80.
- Zhu, M., Riederer, M. and Hildebrandt, U. (2019) UV-C irradiation compromises conidial germination, formation of appressoria, and induces transcription of three putative photolyase genes in the barley powdery mildew fungus, Blumeria graminis f. sp. hordei. Fungal Biology 123, 218-230.