Troy Cowan director, Vision Based Consulting
On May 14, IUVA’s COVID-19 Task Force hosted “Expert Perspectives on UV as a Tool for N95 Decontamination,” a webinar to promote “open dialogue on the use of UV disinfection technologies in combating the COVID-19 pandemic.” The presentations covered Food and Drug Administration, Centers for Disease Control and Prevention N95 disinfection guidelines; COVID-19/SARS-CoV-2; N95 mask basics; and an overview of one example of efficacy testing guidelines. A panel followed, presenting traditional Hg UV bulbs, new technology UV LED sources, healthcare providers, testing labs and the research community. All presenters and panel members then helped respond to questions from an audience of more than 700. More than 100 questions were posed, ranging from UV basics to N95 mask construction to COVID-specific decontamination issues.
 Many thanks to each contributor for sharing expertise and insights into the issues around decontaminating N95 respirators. The slides and video and responses to all questions are being posted to the IUVA website’s COVID-19 section.
Many thanks to each contributor for sharing expertise and insights into the issues around decontaminating N95 respirators. The slides and video and responses to all questions are being posted to the IUVA website’s COVID-19 section.
Here are a few of the many takeaways:
- Regulators often define disinfection and decontamination without regard to their practical testing implications. Disinfection means pathogens are killed by physical or chemical means, while decontamination is defined as cleaning and disinfecting objects sufficiently so they are safe to handle and process. Depending on the definition used for “safe to handle,” the required UV dosage could vary by a factor of three or more and, in some cases, make acceptance testing almost impossible. In its May 2020 guidance revisions, the FDA used only the word decontamination, not disinfection; however, the FDA still required 6-log or greater pathogen reductions, a very difficult requirement to test on N95s, due to geometries and materials, as detailed below.
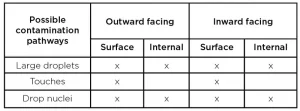
- Because COVID-19’s infection pathways are not clearly understood, it complicates knowing how to best test mask decontamination to assure minimal risk to the user. Three infection pathways were identified: large droplets spread person-to-person; contaminated surfaces touched by a person who then touches their face, etc.; and/or tiny droplet nuclei suspended in air. In all three cases, contamination would be on the mask’s outward face (coming from others), or inside the mask (coming from the wearers). Where and how to best apply the testing inoculate to cover all 10 possible pathways in a calibrated manner (droplet applicators? aerosols? swabs?) and how to best recover the inoculate for analysis are difficult procedural questions that need answers to assure tests are comparable, meaningful and auditable, one lab to another.
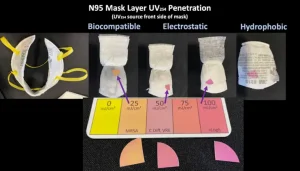
- Testing for internal disinfection of N95 layers is not a straightforward problem. Designing a test to ensure UV penetrates all layers with sufficient dosage levels to disable the pathogens is hard; designing one that can produce adequate and auditable performance results is harder. Again, where to place the inoculates, how much and in how many places for a representative test is uncertain. How to retrieve the samples is even more so.
- Because of differing shapes and construction, not all masks can be treated equally. Fact: UV effectiveness falls off dramatically when it enters at other than a perpendicular angle of incidence, meaning complex mask shapes require UV coming from many angles for credible results. Fact: Folds, corners and overlaps, internally and externally, generate shadows when UV light is applied. Since UV can’t disable what it can’t light up, these shadows cause reduced UV performance. The remedy? Apply a higher dosage to the whole unit. How much more is often a matter of opinion. Some testing labs recommend 150 mJ/cm2, some recommend 500 mJ/cm2 and some recommend 1,500 to 2,000 mJ/cm2, just to be safe. Having no clear standard costs time, money and reduces N95 lifespans.
- Defining basic operational and risk assumptions is key to readily implementable testing. If the risk of reaerosolization of internally embedded contamination is truly negligible, and reuse of masks is restricted to the original user (i.e., Tier Two – no sharing of masks), then tests are only needed for decontamination of exterior and interior surfaces of the mask that could have had surface contamination (no internal layers), regardless of what caused the contamination. This could reduce required doses significantly, possibly down to a range of 500 to 1,000 mJ/cm2 or less. But for these assumptions to be acceptable in an emergency use application, they need to be sanctioned by the CDC and FDA.
- Identification of acceptable biosafety level-2 testing surrogates for SARS-CoV-2 would enhance testing response schedules. Several possible BSL-Two surrogates were discussed, each with advantages and disadvantages; each with reasons for and against, assuming it would be equal to or more conservative in its response to UV compared to SARS-CoV-2. Examples include Surrogate 229E – alpha coronavirus, and OC43 – beta coronavirus, as well as influenza A (H1N1), avian influenza A virus (H5N1), low-pathogenic influenza A(H7N9), avian influenza A (H7N9), MERS-CoV, SARS-CoV, influenza A/PR/8/34, and MS2 bacteriophage, many with existing N95 mask testing histories. Given that SARS-CoV-2 is still being researched to determine its unique set of dosage levels and wavelengths, identifying a suitable surrogate to help promote the industry’s rapid fielding of credible technologies (UV or otherwise) would be a big help.
This dialogue is ongoing. Please join in and contribute your thoughts and experiences.
Through the IUVA Healthcare/UV Working Group, endeavors are being made to promote the acceptance of UV disinfecting technologies as a credible, valued part of environmental management throughout the healthcare industry. In this column, the UV community will be updated on these efforts and the latest information on UV technology as it pertains to the healthcare industry.
Contact: Troy Cowan, troy@visionbasedconsulting.us
References
- IUVA COVID-19 Task Force, Webinar presentations and video, May 14, 2020 (https://iuva.org/Expert-Perspectives-on-UV-as-a-Tool-for-N95-Decontamination-Webinar)
- Ibid.
- ANSI/AAMI ST79:2017, “Comprehensive guide to steam sterilization and sterility assurance in health care facilities“, 2017, Section-2.26
- Ibid., Section-2.23
- Webinar, Blatchley
- FDA, “Enforcement Policy for Face Masks and Respirators During the Coronavirus Disease (COVID-19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff (Revised)”, May 2020, (https://www.fda.gov/media/136449/download )
- FDA, “Recommendations for Sponsors Requesting EUAs for Decontamination and Bioburden Reduction Systems for Surgical Masks and Respirators During the Coronavirus Disease 2019 (COVID- 19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff,” May 2020, (https://www.fda.gov/media/138362/download )
- Webinar, Boyce
- Webinar, Hardwick
- Webinar, Hunt
- Webinar, Hunt
- FDA, “Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings, March 27, 2020 (https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html )
- FDA, “Recommendations for Sponsors Requesting EUAs…,” May 2020
- Webinar, Smith
- Webinar, Hardwick
- Webinar, Blatchley
EXPERT PERSPECTIVES ON UV AS A TOOL FOR N95 DECONTAMINATION WEBINAR
Thursday, May 14 (https://iuva.org/Expert-Perspectives-on-UV-as-a- Tool-for-N95-Decontamination-Webinar)
Webinar Presenters
- “Thoughts on CDC N95 Disinfection Guidelines”– Ernest R. Blatchley III, Ph.D., Purdue Univ.
- “COVID-19/SARS-CoV-2 Basics”– John M. Boyce, M.D., Yale Univ. School of Medicine (ret.)
- “Basics of N95 Masks”– Barry Hunt, Prescientx
- “Overview of ECRI Efficacy Testing Guidelines”– Mairead Smith, ECRI Institute
Webinar Panelists
- Traditional Hg UV Sources – Sam Guzman, American Ultraviolet
- New Technology UV LED Sources – Rich Simons, Ph.D., AquiSense Technology
- Healthcare Providers – Richard Martinello, M.D., Yale Univ. School of Medicine
- Testing Labs – Matthew Hardwick, Ph.D., ResInnova Laboratories
- The Research Community – James Malley, Ph.D., Univ. of New Hampshire