Lia DiMartino
BS, Physician Associate Program, Yale School of Medicine
Richard A. Martinello, M.D.
associate professor, Internal Medicine & Pediatrics-Infectious Diseases, Yale School of Medicine
Over the past 20 years, with the exponential advancements in technology, there has been simultaneous growth in the use of mobile devices such as cellphones, tablets and other personal computing devices. It has reached a point where it is now a societal standard to carry at least one mobile device. The more ubiquitous these devices become, the more users rely on them for everyday tasks. The presence of mobile devices has become pervasive in the healthcare field.
Recent technological developments have permitted mobile devices to play a central role in the clinical setting. These devices aide in interprofessional communication, enhance the efficacy of treatment and allow ready access to an abundance of information, including patients’ medical records – all improving the efficiency and precision of care provided to patients.
As mobile device use has become widespread, the threat a device may pose as a vector for the spread of pathogens must be considered. These devices can become contaminated with pathogens, directly (e.g., splashed with a contaminated fluid) or indirectly (e.g., used with contaminated hands). Without proper decontamination, these mobile devices can become a reservoir for potentially pathogenic bacteria, viruses or fungi. These potential pathogens may be transferred to a healthcare professional’s hand from the device and then can spread to a patient, who may subsequently develop an infection. When studied, mobile devices, not surprisingly, have been shown to be contaminated with several species of microorganisms. Essentially 100% of mobile devices of healthcare professionals are contaminated with bacteria1. Of the bacteria found on those devices, 43.6% were known to cause healthcare-associated infections (HAI)2 in one study.
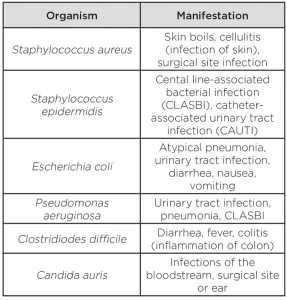
HAIs are defined as infections acquired while receiving medical treatment. HAIs such as ventilator-associated pneumonia, central line-associated bloodstream infection and catheter-related urinary tract infections are associated with the use of invasive medical devices, which are often required for the provision of modern medical care.
These devices include catheters that penetrate the skin and enter blood vessels (central lines), catheters that drain urine from the bladder and tubes that connect critically ill patients to breathing machines (ventilators). These invasive devices have the potential to introduce pathogenic bacteria directly into the patient’s body. The bacteria often responsible for HAIs include Staphylococcus aureus, methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, Pseudomonas aeruginosa, Acinetobacter species, Enterococcus species and Streptococcus species2 (see Table 1).
HAIs have a large impact in the clinical setting, with a high prevalence among hospitalized patients. The Centers for Disease Control and Prevention (CDC) reports that one out of every 31 hospitalized patients develop an HAI, and HAIs not only may prolong patients’ duration of hospitalization but also may lead to death3.
Due to the severity of this problem, there have been public health efforts aimed at reducing the rate of HAIs. Between 2016 and 2017 there was a statistically significant decrease in specific HAIs, between 1% and 13%, among acute care hospitals in the US3. While this is a step in the right direction, much improvement is needed. The rising prevalence of mobile devices in the healthcare setting represents a threat that may reverse this trend.
The contamination of mobile devices is further compounded by the high prevalence of multidrug resistant organisms (MDROs). These are microbes resistant to standard antimicrobial treatment, making treatment of the infection more challenging. These drug-resistant pathogens frequently behave in an “opportunistic” manner and are most often found affecting patients who are already impacted by serious health issues. Such patients constitute a substantial portion of patients in any hospital. Thus, it is imperative to take precautions to limit the spread of these organisms in all ways possible.
So what options are available to sanitize mobile devices? Chemical disinfectants – including isopropyl and ethyl alcohols, quaternary ammonium compounds and accelerated hydrogen peroxides – may be viable options. Many of these chemical disinfectants are commercially available as convenient premoistened, ready-to-use wipes in packaging conducive to use in the healthcare setting. The relatively low cost of these products allows for the convenient placement outside each patient room or in many other areas where cleaning may be performed. By having wipes within reach, healthcare professionals are more likely to comply with decontamination of their cellphones, given the ease and convenience in the busy, fast-paced healthcare setting.
Healthcare staff have grown accustomed to disinfecting their hands upon entry and exit of patient rooms, so requiring cellphone disinfection can be considered a reasonable request. While this method may seem practical, one must also consider the negative aspects of the use of chemical disinfectants. Use of these liquid chemical disinfectants has the potential to damage the mobile device – especially with repeated exposure. Not only can the harsh chemicals be damaging and corrosive, but there is also potential for the liquid to damage the phones, though mobile devices are increasingly more fluid resistant. Even with fluid-resistant mobile devices, staff may remain reluctant to use liquids to clean these devices, particularly if they are personally owned.
Another drawback to note is that disinfectant chemicals require one to even 10 minutes of wet contact time for full microbiocidal action3. It can be challenging to maintain the correct level of wetness even for one minute – decreasing the likelihood that the wipes are being used in accordance with manufacturer instructions. Also, premoistened wipes typically are made of spun polypropylene. This plastic material is not readily biodegradable and further adds to the already substantial environmental footprint of healthcare.
A potential solution to these problems is the use of ultraviolet C (UV-C) light as a means of decontamination, as UV-C light is known to be broadly germicidal. The use of UV-C light eliminates the issues mentioned with chemical disinfectants. There is no exposure to harsh chemicals or liquids that have the potential to damage the device.
While repetitive exposure to UV may solarize the mobile device display and impact other exposed material on the mobile device, extensive exposure may be required prior to any noticeable changes.
Another benefit of avoiding chemicals is that one will be able to use the mobile device immediately following the brief disinfection period. The short disinfection time, typically 20 to 60 seconds, allows the UV systems to align with workflows in busy clinical settings. Finally, light-based sanitization systems provide a passive, hands-free process. This allows the clinician to insert their device and, if need be, complete other brief tasks while disinfection is occurring.
With the growing prevalence of UV-C as a means of decontamination, various UV systems have been developed with aims of decontaminating mobile devices, and there is an increasing use of this equipment in healthcare. Some equipment for UV-C-based disinfection of mobile devices has been shown to be capable of disinfecting as many as four to six mobile devices, two tablets or one laptop in 20 to 60 seconds. Some systems also have USB-charging capability, as well as cloud-based synchronization, which allows devices to be updated and overseen by IT. Studies also have shown that these systems are efficacious in disinfecting other handheld medical devices, such as stethoscopes and hospital ID badges.
The modern, high-tech features of UV disinfection devices make them appealing, but there are also limitations that must be recognized when aiming to integrate such devices into the healthcare setting.
One major barrier, as is seen with the introduction of most new policies, is resistance and opposition, which ultimately can lead to decreased compliance. Based off clinical experience, it is known that many healthcare workers don’t routinely disinfect their cellphones, nor other common equipment, such as stethoscopes. Because this isn’t often emphasized in the current clinical setting, there may be some resistance after introduction of the device when asking people to routinely use it.
In addition, the disinfection systems can be quite expensive. Because of the cost of these devices, it may not be feasible to have multiple systems on one ward. The sparse distribution of these systems in the healthcare setting may limit their use. For example, if the device is in use by one clinician, others may be less likely to wait for it to become available and forgo the disinfection process all together.
Finally, there are some gaps that remain in the complete knowledge of the germicidal properties of UV-C light; however, it is hypothesized that organic materials on the surface of the phone, such as oils from the users’ faces or makeup residue, can interfere with the efficacy of UV disinfection. This is a limitation to UV disinfection that needs to be considered.
To conclude, with the gravity of the threat of MDROs in the setting of high rates of HAI, there is a clear need for more effective methods for mobile device decontamination. The increasing prevalence of these high-impact pathogens has the potential to be detrimental to the health of hospitalized patients.
With the rise in use of mobile devices, there is an increased risk for transfer of pathogens from these devices to patients. It is crucial to develop an effective, efficient way to decontaminate mobile devices in the healthcare setting. It is evident that the benefits of UV-C light and UV disinfection systems may become increasingly prevalent to serve this purpose in the future. The convenience and efficacy of UV systems is appealing and can be optimized if these systems are integrated to the everyday workflow of the healthcare setting.
References
- Murgier J., Coste J.F., Cavaignac E., et al. Microbial flora on cell-phones in an orthopedic surgery room before and after decontamination. Orthopaedics & traumatology, surgery & research. 2016;102(8):1093-1096.
- Sadat-Ali M., Al-Omran A.K., Azam Q., et al. Bacterial flora on cell phones of healthcare providers in a teaching institution. American Journal of Infection Control. 2010;38(5):404-405.
- Centers for Disease Control. www.cdc.gov/hai/data/portal/index.html Accessed October 20, 2019.
Contact: Lia DiMartino, lia.dimartino@yale.edu; Richard A. Martinello, richard.martinello@yale.edu