Roberta Hofman- Caris, senior scientific researcher, KWR Water Research Institute, The Netherlands; Danny Harmsen, scientific researcher, KWR Water Research Institute, The Netherlands
Advanced oxidation processes (AOP) have obtained a lot of attention in literature over recent years. However, not all compounds can effectively be oxidized because, in fact, they already are in an oxidized form. This is true, for example for halogen containing organic compounds (Vellanki et al. 2013), or bromate (Jung et al. 2014, Xiao et al. 2017b). In order to change their properties, like their adsorption on activated carbon surfaces, it might be better to apply a reduction process instead of an oxidation process to make them less hydrophilic (Schoutteten et al. 2016). In recent years, some research has been done in this field. AOPs can be characterized by the production of highly reactive reducing radicals. Often these are generated by applying UV radiation in combination with sulfite, dithionite, sulfide, odide, NaBH4 or Fe2+ ions (Bao et al. 2019, Dong et al. 2018, Duan and Batchelor 2014, Hara et al. 1999, Liu et al. 2014, Xiao et al. 2017b, Xiong et al. 2019, Zhang et al. 2015).
Heterogeneous processes that use n-type semiconductors like TiO2, ZnO, Fe3O4, CdS, SnO3, WO3 etc. also can be used (Xiao et al. 2017b).
Yin et al. (2019) showed that a wavelength of 254 was more effective than 300 or 365 for the reduction of graphene oxide by means of UV/SO3. Yu et al. (2018) and Wang and Liu (2019) showed that the efficiency of the UV/SO3 system can be improved by adding iodide.
Sulfite shows an adsorption maximum at 220 nm, but experiments have been carried out with both LP (253.7 nm) and MP (200 to 300 nm) UV lamps (Duan and Batchelor 2014, Vellanki and Batchelor 2013, Vellanki et al. 2013). Under anoxic conditions, Jung et al. (2014), Xiao et al. (2017a), Liu et al. (2013a), (Yu et al. 2018) and Yazdanbakhsh et al. (2018) present the following reactions that take place when an aqueous sulfite solution is irradiated by UV-C radiation:
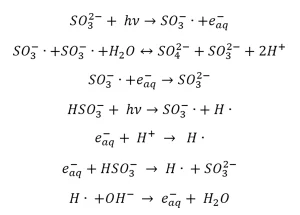
Conversion of organic micropollutants may be caused by direct UV photolysis, by reduction by hydrated electrons or by reaction with the sulfite radicals formed (Li et al. 2019). It was found that natural organic matter (NOM) competes with sulfite for the UV radiation, and, thus, may hinder the conversion of perchlorate (Duan and Batchelor 2014, Vellanki and Batchelor 2013) at relatively high concentrations of compounds, although Jung et al. (2017) observed a higher reaction rate in some cases. This may be caused by the formation of reactive compounds.
Increasing pH values and temperature were found to increase the reaction rate (Liu et al. 2013b, Qiwen et al. 2018). The sulfite concentration shows an optimum, above which mixing problems hindered further increase of reaction rate (Dong et al. 2018, Duan and Batchelor 2014, Qiwen et al. 2018, Vellanki and Batchelor 2013). The hydrogen radical that can be formed can react with chlorinated compounds, resulting in the formation of radicals and Cl2 gas. This effect mainly can be observed at pH values of nine to 11.
Schoutteten et al. (2016) compared the conversion of atrazine, carbamazepine, bromynil, diatrizoic acid and dinoseb by means of ARP and oxidation by ozone. They found ARP results in the formation of other types of products than oxidation. This indeed may result in better adsorption properties (e.g. for dinoseb and diatrizoic acid), but for atrazine and bromoxynil, no effects on adsorption properties were observed, and for carbamazepine even less adsorption on activated carbon was found.
In this work the removal of some metabolites of herbicides (desphenylchloridazone, metazachlor sulfonic acid, metolachloric acid and metolachlor sulfonic acid) by means of a UV/SO3 process was studied. These compounds often are found in sources for drinking water in the Netherlands and Belgium and are difficult to remove by means of e.g. AOP. The effect of oxygen concentration, type of UV lamp and water composition was determined.
Materials and methods
Experiments were carried out in a collimated beam apparatus (CBA) (Hofman-Caris et al. 2012), equipped with either a low-pressure (LP; Philips TUV PL-L95/4P; 95 W HO) or medium-pressure (MP; Philips HOK 20/100; 2 kW) UV lamp.
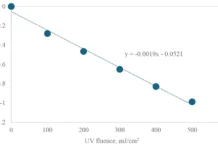
For experiments under low oxygen concentrations, a plastic ring was placed above the petri dish in the CB set-up. Through little holes in this ring, a nitrogen blanket was created above the liquid in the petri dish during experiments. Before the start of the experiment the solution was bubbled with helium for 10 min. In this way, an oxygen concentration of 1.1 mg/L was obtained, which increased to 1.4 mg/L during the experiments. Under atmospheric conditions, the oxygen concentration was about 10 mg/L. A 10 g/L NaSO3 solution was added to the test solution to obtain concentrations of 0, 20, 40 and 60 mg/L, and with UV doses of 0, 300 and 600 mJ/cm2.
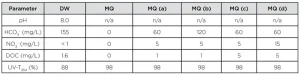
Experiments were carried out in either Milli-Q water or drinking water from the city of Nieuwegein. In order to test the effect of bicarbonate, nitrate and DOC Milli-Q solutions with different compositions were prepared. The water compositions are shown in Table 1.
Results and discussion
The effect of the type of UV lamp used is shown in Figure 1. From these experiments, it can be concluded that both metazachlor sulfonic acid and desphenylchloridazone can be degraded by means of MP photolysis. ARP by means of LP lamps is significantly less effective than by means of MP lamps. Besides, it seems that the presence of oxygen doesn’t always hinder the reaction and actually, in some cases, even seems to enhance the reaction.
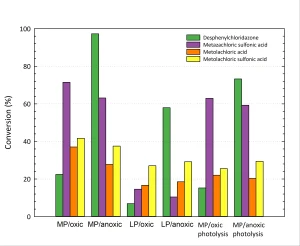
Yazdanbakhsh et al. (2018) showed that the degradation of trichlorophenol, which was mainly attributed to the reaction with sulfite radicals, decreased from 95 to 75% under oxic conditions, but this difference might not prevent application in water treatment. This effect, however, strongly depends on the compounds involved, as shown in Figure 2.
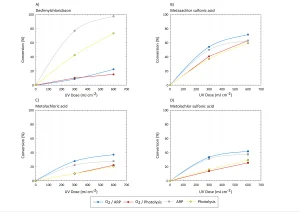
From Figure 2A, it can be concluded that both the photolysis and reduction of desphenylchloridazone are significantly more effective under anoxic conditions than under oxic conditions. For the other compounds, a (slightly) higher conversion is found under oxic condtions. As it will be easier to apply ARP under oxic conditions in drinking water treatment, it was decided to carry out other experiments under oxic conditions.
Furthermore, it seems that the ARP conversion reaches a plateau, which might suggest there is a limiting factor involved. More research will be required to obtain more information about this.
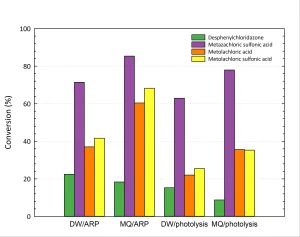
In Figure 3 the effect of the type of water (drinking water or Milli-Q water) is shown.
From Figure 3 it can be concluded that both photolysis and ARP are more efficient in Milli-Q water than in drinking water. This may be due to competition or scavenging by other compounds present in drinking water like nitrate, natural organic matter and bicarbonate. To check this, the separate effects of these compounds were measured, as shown in Figure 4.
These results are a strong indication that the
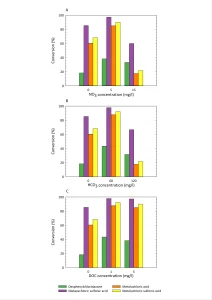
presence of DOC has a positive effect on the conversion of micropollutants. However, there is hardly any difference between 1 and 5 mg/L. For both nitrate and bicarbonate, the presence of a low concentration seems to be advantageous for the conversion in comparison with Milli-Q water, but increasing the concentration further in general seems to hinder the degradation. However, for desphenylchloridazone, the opposite effect seems to occur. It’s not clear yet what causes this effect.
As both the presence of oxygen and NOM seem to enhance the conversion of micropollutants in some cases, the actual reaction mechanism is still unclear. It appears that other reactions than reduction processes may occur too. This will have to be studied further.
Conclusion
From this research it was concluded that ARP may be applicable for degradation of micropollutants in drinking water under oxic conditions. It is possible that under anoxic conditions for some compounds a higher degradation rate is found, but for some compounds the conversion will be in the same order of magnitude. It even seems that the presence of O2 and DOC may enhance the reaction. This raises the question whether the processes observed really are reduction processes, or that e.g. oxygen radicals or organic radicals are involved. MP UV lamps are more effective for this reaction than LP UV lamps.
Acknowledgement
These studies were supported by the Joint Research Program of the Dutch water utilities (BTO).
Contact: [email protected]; [email protected]
References
Bao, Y., Huang, J., Cagnetta, G. and Yu, G. (2019) Removal of F–53B as PFOS alternative in chrome plating wastewater by UV/Sulfite reduction. Water Research 163.
Dong, X., Li, C., Zheng, W. and Wang, G. (2018) Degradation of Polybrominated Aiphenyl Ethers in a UV Advanced Reduction Process with Different Reducing Agents.
Duan, Y. and Batchelor, B. (2014) Impacts of natural organic matter on perchlorate removal by an advanced reduction process. Journal of Environmental Science and Health – Part A Toxic/Hazardous Substances and Environmental Engineering 49(6), 731-740.
Hara, K., Sayama, K. and Arakawa, H. (1999) UV photoinduced reduction of water to hydrogen in Na2S, Na2SO3, and Na2S2O4 aqueous solutions. Journal of Photochemistry and Photobiology A: Chemistry 128(1-3), 27-31.
Hofman-Caris, C.H.M., Harmsen, D.J.H., Wols, B.A., Beerendonk, E.F. and Keltjens, L.L.M. (2012) The Use of Larger Volumes in a Collimated Beam Setup. Ozone: Science and Engineering 34(2), 125-128.
Jung, B., Nicola, R., Batchelor, B. and Abdel-Wahab, A. (2014) Effect of low- and medium-pressure Hg UV irradiation on bromate removal in advanced reduction process. Chemosphere 117(1), 663-672.
Jung, B., Safan, A., Botlaguduru, V.S.V., Batchelor, B. and Abdel-Wahab, A. (2017) Impact of natural organic matter on bromate removal in the sulfite/UV-L advanced reduction process. Water Science and Technology: Water Supply 17(2), 461-471.
Li, C., Zheng, S., Li, T., Chen, J., Zhou, J., Su, L., Zhang, Y.N., Crittenden, J.C., Zhu, S. and Zhao, Y. (2019) Quantitative structure-activity relationship models for predicting reaction rate constants of organic contaminants with hydrated electrons and their mechanistic pathways. Water Research 151, 468-477.
Liu, X., Vellanki, B.P., Batchelor, B. and Abdel-Wahab, A. (2014) Degradation of 1,2-dichloroethane with advanced reduction processes (ARPs): Effects of process variables and mechanisms. Chemical Engineering Journal 237, 300-307.
Liu, X., Yoon, S., Batchelor, B. and Abdel-Wahab, A. (2013a) Degradation of vinyl chloride (VC) by the sulfite/UV advanced reduction process (ARP): Effects of process variables and a kinetic model. Science of the Total Environment 454-455, 578-583.
Liu, X., Yoon, S., Batchelor, B. and Abdel-Wahab, A. (2013b) Photochemical degradation of vinyl chloride with an Advanced Reduction Process (ARP) – Effects of reagents and pH. Chemical Engineering Journal 215-216, 868-875.
Qiwen, M., Kim, K.H. and Han, I. (2018) Correlation analysis of pollutant factors influencing the sulfite/UV-L advanced reduction process. KSCE Journal of Civil Engineering 22(2), 475-481.
Schoutteten, K.V.K.M., Hennebel, T., Dheere, E., Bertelkamp, C., De Ridder, D.J., Maes, S., Chys, M., Van Hulle, S.W.H., Vanden Bussche, J., Vanhaecke, L. and Verliefde, A.R.D. (2016) Effect of oxidation and catalytic reduction of trace organic contaminants on their activated carbon adsorption. Chemosphere 165, 191-201.
Vellanki, B.P. and Batchelor, B. (2013) Perchlorate reduction by the sulfite/ultraviolet light advanced reduction process. Journal of Hazardous Materials 262, 348-356.
Vellanki, B.P., Batchelor, B. and Abdel-Wahab, A. (2013) Advanced reduction processes: A new class of treatment processes. Environmental Engineering Science 30(5), 264-271.
Wang, L. and Liu, X. (2019) Fast degradation of monochloroacetic acid by bioi-enhanced UV/S(IV) process: Efficiency and mechanism. Catalysts 9(5).
Xiao, Q., Wang, T., Yu, S., Yi, P. and Li, L. (2017a) Influence of UV lamp, sulfur(IV) concentration, and pH on bromate degradation in UV/sulfite systems: Mechanisms and applications. Water Research 111, 288-296.
Xiao, Q., Yu, S., Li, L., Wang, T., Liao, X. and Ye, Y. (2017b) An overview of advanced reduction processes for bromate removal from drinking water: Reducing agents, activation methods, applications and mechanisms. Journal of Hazardous Materials 324, 230-240.
Xiong, Z., Zhang, H., Zhang, W., Lai, B. and Yao, G. (2019) Removal of nitrophenols and their derivatives by chemical redox: A review. Chemical Engineering Journal 359, 13-31.
Yazdanbakhsh, A., Eslami, A., Moussavi, G., Rafiee, M. and Sheikhmohammadi, A. (2018) Photo-assisted degradation of 2, 4, 6-trichlorophenol by an advanced reduction process based on sulfite anion radical: Degradation, dechlorination and mineralization. Chemosphere 191, 156-165.
Yin, R., Shen, P. and Lu, Z. (2019) A green approach for the reduction of graphene oxide by the ultraviolet/sulfite process. Journal of Colloid and Interface Science 550, 110-116.
Yu, K., Li, X., Chen, L., Fang, J., Chen, H., Li, Q., Chi, N. and Ma, J. (2018) Mechanism and efficiency of contaminant reduction by hydrated electron in the sulfite/iodide/UV process. Water Research 129, 357-364.
Zhang, C.J., Qu, Y., Zhao, X. and Zhou, Q. (2015) Photoinduced Reductive Decomposition of Perflurooctanoic Acid in Water: Effect of Temperature and Ionic Strength. Clean – Soil, Air, Water 43(2), 223-228.