By Danny Bayliss, Campden BRI; Keith Warriner & Mahdiyeh Hasani, Department of Food Science, University of Guelph; Tatiana Koutchma, Agriculture and Agri-Food Canada
In this article, members of the IUVA Food & Beverage Committee reviewed and summarized the historical perspective and updates to regulations and standards related to the application of UV for water, food and beverages.
Update on European Regulations and Standards
The concept of a European Union (EU) first was considered in 1920, although it took WWII to realize that this could be achieved by more diplomatic means. The treaty of Rome was signed in 1957 with core countries of Belgium, France, Italy, Luxembourg, the Netherlands and West Germany. The United Kingdom (UK) initially was given the cold shoulder but then finally entered in the 1970s, and other countries subsequently joined the club. The point of relevance is that each member state had existing food regulations, and the EU centre had little influence given it was more focused on facilitating cooperation than regulations. However, the EU progressively introduced regulations; at the same time, member states could maintain their own food safety regulations given the historic base. This has led to disputes between members – to the point that some left, such as the UK in Brexit. The main point to consider is that there are differences in how UV technology is viewed and the associated regulations that need to be followed.
UV treatment in Europe
Germany has a local regulation on treatment of food with electron, Gamma and X-rays, neutrons or ultraviolet rays (Food irradiation regulation – LMBestrV) Germany/Europa. In their approvals section (4), treatment by direct exposure to ultraviolet rays is permitted for sterilization of
- drinking water,
- the surface of fruit and vegetable products and
- hard cheese during storage.
- Indirect ones occurring in the sterilization of air by ultraviolet rays’ exposure to food is permitted.
For some food product specifically, it has been noted that UV might not be permissible in the EU depending on the status of the technology and local state legislation. There is some retained Regulation (EU) 2019/627 1 in the UK, where Article 45 states: ‘The official veterinarian shall declare fresh meat unfit for human consumption if it: l) has been treated illegally with ionising radiation, including UV-radiation.’
Exploring this with the relevant food agency highlighted there were no current food safety issues known to them using UV and there also is no UK legislation which prohibits the treatment of food with UV technology. As it is not illegal to use the technology, then meat applications could, in principal, be permitted in the UK market. This does not translate to all other EU countries as the specifics of their local legislation need to be understood.
It also should be noted that the use of UV does not replace good hygiene practices and visible contamination still must be removed, as set out in other parts of the food legislation (Retained regulation 2004/853 2). The manufacturer looking to use UV radiation also is responsible for ensuring it is suitable, effective and safe, conforming with general food safety and hygiene requirements (Retained regulation 2004/853 2 and Regulation 2017/625 3). Food safety management plans should be updated to reflect the use of this method, if implemented.
Generally, food-based applications are using the technology for decontamination, where the manufacturers are setting a target they would like the technology to achieve to improve the safety or maintain the safety of their products based on the associated risks or extend product shelf-life. Food manufacturers will test to see if the treatment causes any changes in the product that would not be accepted by the consumer and the nutrients are not compromised. In most cases, the technology does not substantially change the product and, therefore, does not require EU Novel foods approval. It is only when exposure of the UV causes substantial change (positive or negative) in the product that approval is necessary. Approvals have been granted for mushrooms and mushroom powder, yeast and bread and milk applications because of a boost in a vitamin D as a result of the exposure. Care must be taken to stay within the right tolerances for these applications because too high a vitamin content in daily consumptions can have health implications, which is considered in the novel food assessments.
More recently, a lot of emphasis – particularly with COVID-19 – was placed on using UV devices for disinfection. Disinfection comes with pre-defined log reductions that have to be achieved in order to determine if a chemical or device is suitable for disinfection. This typically is 4 log reductions for selected bacteria and 3 log reductions for yeast and molds. As a result of lack of standardization for the technology, the European disinfectant standard writing body CEN released a standard in 2022 that covers the requirements and methodology for testing the efficacy of UV devices (BS 8628:2022). The standard is titled:
BS 8628:2022 Disinfection using ultraviolet radiation. Methods for quantitative testing of automated ultraviolet disinfection activities by direct illumination. Determination of bactericidal, mycobactericidal, sporicidal, yeasticidal, fungicidal, virucidal and phagocidal activities
This new standard has been based on the existing airborne surface disinfection standard EN 17272:2020 for the preparation of the organisms and coupons for treatments and sample recovery. Stainless steel carriers are inoculated with a suspension of microorganism and interfering substance (simulating clean or dirty conditions) in triplicate for each organism and dried onto the surface. When ready, test samples are moved into the treatment room or chamber, which is blacked-out. The chamber must be equilibrated for temperature (as standard 20 ± 1 ° C) and relative humidity (30-60%) before starting the cycle.
Test samples are placed horizontally over a flat black matte surface at a specified height, with the inoculum facing up. The UV system then is placed within the room or chamber at a specified distance from the sample stand and the disinfection cycle is started. The total UV time is recorded once the lamps are switched on until they are switched off. This time will be set by the equipment manufacturer to ensure disinfection of the test coupons and specific microorganisms. The intended application setting (food, veterinary or hospital) will influence the organisms used for the trial.
The carriers then are recovered in a liquid medium via agitation with glass beads and plated out on suitable agar plates. The microbial colonies are counted after a suitable incubation period, and the treatment efficacy is expressed in LOG reductions. Greater than 4 logs is required for bacteria and 3 logs for yeast and molds.
Conclusions
Member states of the EU are protective of their own food safety regulations, and these need to be appreciated when introducing UV-based technologies. This does not only relate to the country in which the technology is applied but also to where the food processor exports its product. An overriding philosophy of the EU is to take the precautionary principle to food safety. Specifically, for a technology to be introduced, it should be demonstrated that no harmful chemicals are produced (for example, photoproducts) in the food due to the treatment. Proving negatives can be a challenge, which is why the EU food safety system is more rigorous than in North America. Yet, UV has an advantage in this regard given the move away from synthetic chemicals.
Update on North American Regulations and Standards
Ultraviolet treatment of water and food/non-food surfaces has a long history of proven efficacy. Yet, the commercial application of UV-based technology remains underutilized for treating liquids, foods and non-food surfaces. Part of the reason can be attributed to the regulatory hurdles that need to be overcome to obtain no objections or approvals for UV-based processes. Although regulatory changes move slowly, there have been revisions, and opportunities, for UV-based technologies within the food sector.
A brief history of UV-related food safety regulations
Within North America, there is a confusion over the use of UV as a decontamination method for foods. The confusion originated from the US Food, Drugs, and Cosmetic Act of 1958 that defined irradiated products as a food additive, thereby requiring labeling. Given that UV is part of the electromagnetic spectrum, it too was considered like irradiation and, consequently, foods treated with ultraviolet had to be labeled as such. It could be envisaged that back in 1958 the reason for inclusion of UV as irradiation was more related to closing loopholes in regulations rather than having a scientific base. Nevertheless, the legislation has held back the application of UV, in addition to irradiation in general, despite the obvious benefits to public health in reducing the burden of foodborne illness. This was recognized by the FDA, which in 1999 introduced a proposal to remove the labeling requirements given that it resulted in ‘consumer anxiety.’ However, there was push-back from consumer groups, which suggested removing the label was against the public interest and thus the labeling requirement remained.
Later in 2007, a final rule was proposed by the US FDA whereby no labeling was required to foods in which irradiation treatment did not cause any material changes to the product. Here, a submission was, and is, required to the FDA, which then is assessed on a case-by-case basis. Essentially, the FDA review would judge if the UV treatment resulted in changes to the product – be it positive or negative (in which case, it would be categorized as a Food Additive). However, if no positive or negative changes occurred to the product by UV treatment while having a purpose (e.g. reducing microbial loads), it will be designated a Processing Aid with no need to label. This was the reason why UV treatment can be applied for treating fruit juices without the need for labeling with the irradiation symbol.
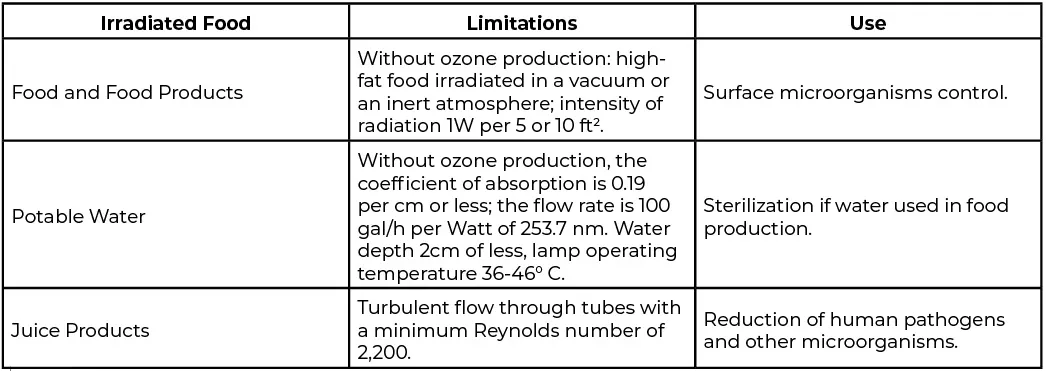
Subsequent applications have been made for treating grains, cheeses, baked products, frozen foods and liquid egg. The key is that products are considered on a case-by-case basis in terms of efficacy and lack of material changes proven. The FDA does not stipulate a lower or higher limit for UV dose – only that it be generated by a low-pressure lamp and not cause the formation of byproducts. However, even if considered a processing aid, applying UV for treating organic foods is restricted. In addition, foods treated with UV in Vermont require labeling, although no other US state or province within Canada requires such foods to do so. Regulations related to UV treatment of water and foods is covered in the Code of Federal Regulations’ (CFR) 21CFR179.39. Within the regulation, only low-pressure lamps emit 90% of the emission at 253.7 nm, with limitations outlined in Table 1.
It should be noted that a few UV light treatment systems for juice products and beverages were developed and built in the USA, Europe and Israel that meet these FDA rule. However, their practical implementation still is slow due to the need of established validation approaches to comply with juice HACCP. Also, a revision of Pasteurized Milk Ordinance (PMO) in 2017 approved the application of monochromatic and polychromatic mercury lamps for sterilization of water for washing dairy equipment. This rule established the UV intensity of 186 mW/cm2 at 254 nm for low-pressure lamps and 120 mW/cm2 for medium-pressure lamps to provide equivalent of pasteurization or 4-log reduction of adenovirus.
Alternative light sources, such as Pulsed Light that has additional wavelengths to 254 nm, have been approved for decontaminating food surfaces. The limitations are that xenon lamps are covering 200-1000 nm and operated with a duration no longer than 2ms with a maximum dose of 12J/cm2. There currently is no regulatory guidance on Far UV or UV LEDs, with any uses having to be approved via a food-additive application.
Pre-harvest water standards are revised again
One of the first Final Rules (regulations) introduced under the Food Safety Modernization Act (FSMA) was related to fresh-produce safety, given the numerous outbreaks linked to fruit and vegetables. It has long been known that irrigation water represents a significant source of human pathogens, and it was required to be monitored and interventions applied. The focus was placed on irrigation water microbiological criteria that initially was set for standards used for recreational water, given that the portable standard was considered too stringent. Yet, with the continuing foodborne-illness outbreaks linked to fresh produce, the standards were revisited in May 2024 with the amended 21 CFR Part 112 regulation. In the revised regulations, introduced in May 2024, the microbiological standards have been revised to no E. coli detected in 100 ml samples to be in line with the criteria for potable water. Yet, there are no testing requirements within the regulation. That indirectly means it is up to the grower to decide on the frequency of testing. However, there is mention that the grower is required to consider implementing a risk management intervention (i.e., water disinfection method), as described in 112.46.
Although UV or any other treatment is not specified, the regulation is outcome-based in that the treatment must be monitored and consistently achieve no detectable generic E. coli/100 ml. Although not officially specified, a 3 log CFU reduction of E. coli is defined as an effective treatment. UV treatment of water has a long history, although it has been met with variable performance with respect to disinfection of irrigation water. The variability is attributed to the diverse range of turbidity/absorption coefficients encountered in water sources, which researchers in the area seldom consider. Notably, there is little consideration of pre-treating the water to increase the UV transmission to ensure a consistent UV process. Currently, peracetic acid and chlorine are being promoted for pre-harvest water, despite issues relating to disinfection byproduct formation and sequestering. Consequently, there is a need to revisit the treatment of irrigation water with UV light, with the range of water characteristics (e.g. turbidity) being determined.
UV and traceability
The final traceability rule (Section 204d) was introduced in June 2024 to meet the FDA mandate of Smarter Food Safety. The rule enabled the traceability of foods from the farm to the fork, with the consideration that contaminated products rapidly can be identified and hence removed, leading to reduced impact of foodborne-illness outbreaks. Critics have cast doubt on how effective traceability will be in reducing illness given that many outbreaks are identified when little product remains on the market. Nevertheless, the regulation requires processors, packers and distributors to identify Key Data Elements (i.e., key information) and Critical Tracking Events (points in the chain) that can be provided to the FDA within 24 hours upon request.
Traceability is challenging by its very nature, given that raw materials are sourced from various origins and often co-mingled. Further complexity is added when multiple ingredient products are prepared and widely distributed. As part of the amendment to the traceability rule, it is possible to reduce the length of the chain by introducing an intervention step to reduce relevant pathogen by 5 log CFU. For example, in the case of table eggs, it is possible to limit the traceability requirement from the point of disinfection rather than needing to trace back to the farm. This reduces the complexity of traceability and associated costs. UV and UV-based treatments (e.g. hydroxyl-radical treatment) can be applied to table eggs to achieve the 5-log CFU reduction of Salmonella. Therefore, there is a strong incentive to adopt intervention technologies, especially UV, which has advantages over other non-thermal intervention technologies.
Conclusions
When first introduced, food-safety regulations were intended to set standards to provide assurance to the consumer that there had been oversight over production-processing and distribution. Over the years, it could be argued that regulations have become too complex and restrictive in introducing new technologies. This is further complicated by regional differences, such as those applied within Vermont. Although this may deter the introduction of technologies such as UV, it should be noted that many regulators are open to considering food-safety interventions, the process for consideration is relatively straightforward and it can bring about change in the regulations for the better.
References, European
- Retained regulation 2019/627, https://www.legislation.gov.uk/eur/2019/627/contents
- Retained regulation 2004/853, https://www.legislation.gov.uk/eur/2004/853/contents
- Regulation 2017/625, https://www.legislation.gov.uk/eur/2017/625/contents
Useful links, North American
- FSMA Final Rule on Pre-Harvest Agricultural Water, https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-pre-harvest-agricultural-water#:~:text=The%20final%20rule%20includes%20a,the%20outcomes%20of%20the%20assessment
- FSMA Final Rule on Requirements for Additional Traceability Records for Certain Foods, https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-requirements-additional-traceability-records-certain-foods
- CFR – Code of Federal Regulations Title 21, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=101.100






