By Liz Stevens, writer, UV Solutions; and Lou Ann Tom, Ph.D.
Lou Ann Tom is a researcher on a mission. Tom, associate professor of chemistry at Susquehanna University, Selinsgrove, Pennsylvania, studies methods for detection of pharmaceuticals in waterways as well as methods for the photodegradation of those pharmaceuticals. She also is active in part of a medication take-back group at Geisinger Medical Center, Danville. This group has created a medication take-back program designed to reduce the number of discarded pharmaceuticals that could be released into the environment.
Identifying and mitigating the incidence of pharmaceuticals in waterways is of concern not just to Tom, but also Geisinger and Tom’s Pennsylvania community. It is of concern to stakeholders worldwide because an increasing amount of discharged, discarded and flushed drugs enter the environment and, especially, waterways. According to the IQVIA Institute’s report, “The Use of Medicines in the US,” 6.3 billion prescriptions were dispensed in the nation in 2020.1 During the US Drug Enforcement Administration’s semi-annual National Take Back Day in October 2021, approximately 372 tons of returned prescriptions were collected,2 which likely represents a mere fraction of the meds consumed and discarded by Americans. Geisinger’s medication take-back program has collected over 21 tons of returned medications at more than 40 collection sites since 2018.
Pharmaceuticals that may be toxic to the environment are not limited to those used for human medicine; they also include those used in veterinary medicine for pets and for treating agricultural livestock. The more pharmaceuticals that are developed, manufactured and distributed, the more the potential for pharma residue to enter the environment at large. What is good for the patient may end up being harmful for the flora and fauna (including humans) in the spent pharma’s effluent trajectory.
Understanding potential impact
In 2010, a United States Geological Survey study revealed that discharge from pharmaceutical manufacturing facilities can be a significant source of the pharmaceutical levels found in downstream water samples,3 but that’s not a big surprise. Since then, additional targeted surveys and studies have confirmed these findings and have shown that the sources and effects of pharmaceuticals in waterways actually are greater and are compounded by the tendency of pharma chemicals to be altered in the environment.
A survey of water samples from 258 rivers in 104 countries – representing the “pharmaceutical fingerprint” of 471.4 million people – found contaminants in surface water that pose a threat to the environment and/or human health in more than a quarter of the locations studied.4 Research also has revealed that, in addition to the expected effects of pharmaceuticals in water supplies, surprising effects – including increased toxicity – can develop when compounds degrade and turn into new compounds in the presence of water, sunlight, microbes and soil.5
Municipal wastewater treatment plants are not designed to tackle the complex job of neutralizing pharma chemicals, and they would not address the veterinary use “residue” that reaches the urban/suburban landscape or the effluent from livestock that then may seep into rural waterways. Clearly, a variety of comprehensive approaches targeting the various points of “discharge” needs to be researched, designed and implemented.
The professor joins the effort
Lou Ann Tom’s background set the stage for her current research efforts. Prior to her role at Susquehanna University, which began in 2007, Tom was a chemist/senior scientist at pharma giant Merck & Co., Inc. When Merck developed ivermectin, a drug that is relatively toxic to the environment, Tom researched methods to improve the detection limit of analytical techniques for detecting the drug at very low concentrations, at which the drug can be a health issue for freshwater organisms.
Tom’s search for a suitable testing method led her to molecularly imprinted polymers (MIPs), which can be used to test for ivermectin and other compounds that could be toxic at very low levels. With MIPs, Tom created polymers engineered to have specific cavities in the matrix to which the selected target molecule of interest has an affinity. With a filter made of such polymers, one can concentrate molecules from water for further analysis via high-performance liquid chromatography. A key aspect in creating the MIPs, however, is to be careful with the ultraviolet (UV) dose used to initiate polymerization: the right or, rather, the wrong UV dose will cause the compound of interest to degrade before the polymer can be completed.
After leaving Merck and joining the faculty at Susquehanna University, in addition to methods for identifying minute concentrations of compounds in water, Tom expanded her research to include degradation methods for compounds. Tom’s background at Merck and her knowledge that some pharma compounds are susceptible to UV degradation – combined with published literature from other research centered on using UV for this purpose – led Tom to using UV two-fold. UV is first used to build the polymer technology for detecting minute concentrations of toxic compounds, and then UV is used to destroy/dismantle the compounds – UV, as both photoinitiator and phototerminator. Tom’s current slate of molecularly imprinted polymers includes those for warfarin, oxycodone hydrochloride, bifenthrin, esfenvalerate and other drugs and pesticides of concern.
UV to zap toxicity
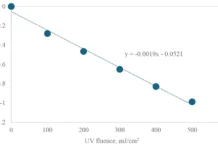
Lou Ann Tom’s research at Susquehanna to degrade the drugs has involved using UV sources at 254 nm and 300 nm for a few hours to a few weeks. Her equipment includes a Rayonet model RMR-600 UV mini reactor with eight vertical UV lamps surrounding the sample of the drug to be degraded. She has found that UV at 254 nm is the more effective wavelength. She envisions a future in which drop-off sites for unused pharmaceuticals could be outfitted with similar UV treatment to degrade the drugs before discharge.
While the UV treatment alone seems to work very well for water-soluble pharmaceuticals (with degradation in hours), water-insoluble compounds take much longer, with degradation sometimes not significant after days or weeks of exposure to UV light. To address this problem, Tom is looking at using various catalysts to increase the rate of degradation.
In conjunction with Dr. Travis Tasker, assistant professor of environmental engineering at St. Francis University, Loretto, Pennsylvania, Tom now is studying the catalytic effect of adding iron oxide (from acid mine drainage) to the samples during UV treatment. Research has shown that the presence of zinc oxide, titanium (IV) oxide or hydrogen peroxide can enhance the UV degradation of compounds such as ibuprofen via an advanced oxidation process.6
As pharma companies work to mitigate the effect of their manufacturing discharge into the environment, the agricultural pharma players and their users work to keep pharma runoff out of fields and streams. Nationwide, pharma return programs organized by communities and law enforcement agencies are being expanded for pharmaceutical take-back locations, and research like Lou Ann Tom’s is being developed to improve options for drug disposal.
References
- Institute Report, “The Use of Medicines in the U.S.: Spending and Usage Trends and Outlook to 2025,” May 27, 2021. https://www.iqvia.com/insights/the-iqvia-institute/reports/the-use-of-medicines-in-the-us.
- “October 2021 National Take Back Day Results,” United States Drug Enforcement Administration, https://www.dea.gov/takebackday#results.
- Water Science School, “Pharmaceuticals in Water,” United States Geological Survey, June 6, 2018. https://www.usgs.gov/special-topics/water-science-school/science/pharmaceuticals-water.
- John L. Wilkinson et al., “Pharmaceutical pollution of the world’s rivers,” Proceedings of the National Academy of Sciences (PNAS), February 14, 2022. https://www.pnas.org/doi/full/10.1073/pnas.2113947119.
- Kimberly M. S. Cartier, “Drugs in Our Water Can Leave Even More Toxic By-Products,” Eos, December 10, 2019. https://eos.org/articles/drugs-in-our-water-can-leave-even-more-toxic-by-products.
- Zhao Wang et al., “Degradation of Ibuprofen by UV-LED/catalytic advanced oxidation process,” Journal of Water Process Engineering, Volume 31, October 2019. https://doi.org/10.1016/j.jwpe.2019.100808.