Rich M. Simons, Ph.D., head of application science, AquiSense Technologies
Ernest R. Blatchley III, Lee A. Reith professor in environmental engineering, Purdue University
Karl Linden, Ph.D., University of Colorado Boulder, Linden Research Group
UV-C radiation in the wavelength range 200 to 225 nm (i.e., far UV-C radiation) has received public attention for claims of disinfection capabilities and safety of human skin and eye exposure; this is contrasted to conventional germicidal ultraviolet irradiation (UVGI) in the wavelength range 240 to 280 nm, which is well accepted as an effective disinfectant but also known to be hazardous to human skin and eyes under direct exposure.
Lamp technologies covering both spectral ranges have existed for many decades, though far UV-C has not been widely applied.
Laboratory studies have shown that far UV-C sources can inactivate microorganisms at comparable rates to, or faster than, conventional UVGI sources.
The two technologies share common disinfection pathways, where direct photolysis of DNA/RNA and/or proteins inactivates the target pathogen. Radiation across the UV-C spectrum is a known disinfectant, though the volume of research on far UV-C disinfection is substantially smaller than that of mature UVGI technology.
“Skin safe” claims for far UV-C are based on the concept that the higher energy radiation at these shorter wavelengths is absorbed by outer protective skin cells (stratum corneum), or in the case of eyes, the outer tear layer, and therefore does not reach susceptible tissue to cause damage.
By contrast, bacteria and viruses do not have such shielding and are directly exposed. Skin and eye safety claims are supported by several studies in live mouse models, mouse tissue and human tissue models with promising findings. Though more work is required, the evidence suggests the absence of conventional skin damage indicators that would typically result from UVGI.
To date, no clinical or long-term studies of human exposure have been conducted, and the effect on injured skin or eyes is unknown. Such work is essential in determining the safety of this technology before application is widespread.
While initial findings are positive, further investigations are required on any secondary impacts of the technology when used in the presence of humans. For instance, the potential for unexpected photochemical reactions, e.g. in cosmetics or clothing; the potential for generation of ozone during continual operation or within enclosed spaces; and determination of threshold limit values (TLVs) for safe daily exposure are all topics that need to be better understood.
Far UV-C is a promising technology application, though currently in the early stages of research. There is not yet sufficient evidence to support widespread application where direct human exposure is anticipated. The IUVA recommends far UV-C not be implemented as an unshielded disinfection technology until sufficient evidence for safety is presented, and suitable protocols for application are established.
Scope
This white paper was produced by a working group of the International Ultraviolet Association (IUVA). This particular working group consists of IUVA members ranging from UV equipment manufacturers, scientists and engineers, to consultants and members of the medical profession.
The objective of this work is to provide an impartial presentation of the available facts on the technology commonly referred to as far UV-C and an analysis based on expert interpretation and knowledge of the field of UV disinfection, safety and public health. The IUVA does not endorse any private entities discussed herein, nor will it pass comment on the accuracy or validity of claims made by individual vendors.
The UV spectrum and far UV-C
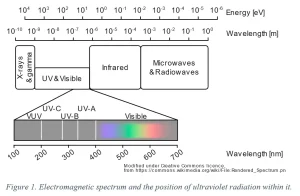
The ultraviolet spectrum (Image 1) is a band of electromagnetic radiation at higher energies than visible light, split into four major categories: UV-A (400 to 315 nm), UV-B (315 to 280 nm), UV-C (280 to 200 nm) and vacuum-UV (VUV, 100 to 200 nm). Far UV-C is a sub-classification of UV-C in the 200 to 225 nm range.
Disinfection by ultraviolet radiation
UV-A and UV-B are present in sunlight at the earth’s surface; these parts of the ultraviolet spectrum are common causes of sunburn and, with longer-term exposure, various forms of skin cancer including melanoma. The risks of human exposure to UV-A and UV-B are well known. Solar UV may be used for disinfection purposes (SODIS), where exposures on the order of several hours to days can be effective at treating surfaces and water. Artificial sources of UV-A and UV-B are not commonly used for disinfection.
UV-C radiation, and more specifically germicidal ultraviolet in the 240 to 280 nm range (UVGI), has been used for pathogen disinfection for over a century, with applications in water treatment, in air systems and on surfaces. The use of UVGI as a disinfectant is supported by decades of scientific research. UV-C radiation is absorbed by DNA and RNA (the genetic code for all lifeforms), changing its structure (Jagger 1967; Harm 1980).
This damage inhibits the ability of the affected pathogens to reproduce, meaning that they cannot infect and are no longer dangerous. Whereas the UV exposure required to inactivate different microorganisms varies, there are no known microorganisms that are immune to this treatment; UVGI is regularly used against bacteria, viruses and protozoa. Far UV-C radiation functions in a similar manner to UVGI, though the absorption by proteins in this wavelength range may play a larger role in inactivation (Taylor 2020).
VUV is not commonly used for disinfection. High-energy VUV photons break apart oxygen molecules in air, ultimately leading to ozone formation, which causes irritation of the human respiratory system and some external tissues. This chemical by-product limits air and surface disinfection application of VUV. In water treatment applications, the strong absorption of VUV by water can limit reactor efficiency. Similar effects are also observed in short wavelength UV-C radiation, along with the attendant limitations to application.
UV disinfection is controlled by two key parameters: wavelength and total UV exposure (commonly referred to as UV dose). Germicidal efficacy varies with UV-C wavelength and is unique to the pathogen being targeted – the germicidal action spectrum describes this response. Though germicidal action spectra vary among microbial species (Beck 2015), a common feature is a local peak in the 260 to 270 nm region. Hence the historic focus of conventional UVGI systems. Wavelengths below 230 nm typically are highly effective for inactivation but have been less widely researched.
Krypton chloride excimer (KrCl*) lamps with peak emission of 222 nm are the most commonly studied far UV-C range sources.
Studies have shown KrCl* lamps to be effective against a wide range of pathogens, including viruses such as influenza (H1N1), alpha and beta coronaviruses, adenovirus (Welch 2018, Buonanno 2020, Beck 2014; Hull and Linden 2018) and vegetative bacteria such as Staphylococcus aureus, Escherichia coli and Bacillus subtilis (Clauss 2009, Matafonova 2008).
For a given wavelength, the degree of inactivation (measured as log reduction) is governed by the UV dose, being the product of irradiance (UV power per unit area) and exposure time. The design of a disinfection system must define a microbial target and a desired degree of disinfection. Decades of dedicated research has provided a large database correlating conventional UVGI dose to degree of disinfection (Malayeri 2016).
Though data exists for far UV-C, it is relatively sparse and requires more careful definition of required doses for inactivation. Like conventional UVGI, the efficacy of far UV-C is species-dependent, and each application must be assessed based on its requirements.
Though the capability of far UV-C radiation as a disinfectant is well demonstrated, validation of its performance in field applications is generally lacking. For conventional UVGI, protocols have been agreed on by academia, industry and regulators for the validation of systems prior to use. This allows for safe and effective application of UVGI. However, no such accord exists for far UV-C application, and bespoke protocols must be defined to assess manufacturers’ claims.
Sources of far UV-C radiation
UV-C disinfection relies on the generation of radiation from artificial sources. The most common far UV-C sources are KrCl* excimer lamps (tubular or micro-plasma type), in which a voltage applied across a sealed chamber containing the gas mix causes spontaneous generation of UV-C photons.
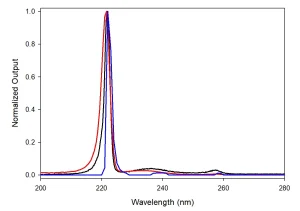
Emission spectra from KrCl* lamps show a dominant peak at 222 nm with full-width-half-maximum ~4 nm and often a long-wavelength “tail” through the UVGI UV-C range. These off-nominal emissions represent ~5% of the total power output of a typical unfiltered KrCl* lamp. Though optical filtering can be used to limit emissions outside of the 222 nm peak, excimer sources are not monochromatic (Image 2), and the full emission spectrum must be considered when evaluating their safety.
Safe for human exposure?
UV-C radiation is absorbed by biomolecules and can damage cellular processes. However, multicellular organisms that have evolved environmental protection mechanisms, such as the stratum corneum in humans, may be shielded from these effects by absorption of harmful radiation by these means (Buonanno 2013, Brenner 2017).
The biophysical rationale for the “skin safety” of far UV-C follows exactly this principle, i.e., that the harmful radiation cannot reach the live skin cells and thus cannot cause harm.
Ocular damage (e.g. photokeratitis) under UV-C exposure is also a concern, though it is claimed that the tear layer affords a similar protection to the live cells (cornea) underneath (Welch 2018). The authors were not able to identify an evidence base for this claim within the literature.
Chronic and acute exposure to far UV-C has been studied in mice and human skin models (Narita 2018, Buonanno 2017, Yamano 2020).
These works focus on 222 nm radiation (from optically filtered KrCl* sources) and observation of any conventional UV damage indicators, such as DNA lesions. Sources of 207 nm and wider skin damage indicators also have been considered, including indicators for skin inflammation and differentiation.
Protein damage is known to be more prevalent at shorter UV-C wavelengths (Beck 2018), resulting in viral capsid damage and inhibition of viral infectivity (Vazquez-Bravo 2018); however, more work is required to build a comprehensive understanding of this process.
One in vivo study of human skin exposure is known in the literature (Woods 2015). The study used a KrCl* excimer lamp provided by the manufacturer, without any additional optical filtration. Erythema and DNA damage were observed at moderate-to-high irradiation levels (40 to 101 mJ/cm-2).
The article claims that such exposure is below that required for disinfection, though more recent literature (cited above) indicates that UV doses in this range could result in meaningful disinfection. Due to the impure spectrum of this source, any effects from the far UV-C radiation could not be decoupled from those of the longer-wavelength UV-C radiation.
The study is, therefore, neither evidence for nor against the safety of far UV-C radiation for humans. However, it is a strong indication of the need for careful consideration of the technology when discussing potential human exposure.
Common limits are agreed for the maximum daily exposure to artificial UV-C by the International Committee on non-Ionizing Radiation Protection (ICNIRP 14/2007), American Conference of Governmental Industrial Hygienists (ACGIH 2008), European Commission (2006/25/EC), American National Standards Institute/Illuminating Engineering Society (ANSI/IES RP-27.1-15) and International Electrotechnical Commission (CEI/IEC 62471:2006).
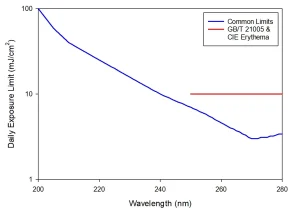
Agreement on a spectral weighting and overall exposure limit allows for these standards to be summarized into Image 3, showing the expected daily (eight-hour equivalent) safe exposure limit as a function of wavelength across the UV-C spectrum. It should be noted that these limits are set for a maximum unintentional exposure and are not intended to define repeated exposure guidelines.
Consider these limits in terms of an acceptable exposure to even a low irradiance of 0.1 mW cm-2: the maximum exposure of a 254 nm source would be one minute and less than four minutes for a 222 nm source. Though expected safe exposure limits of far UV-C are higher than those of conventional UVGI, far UV-C remains widely considered to represent a skin and eye damage hazard to be managed.
An outlier to the agreed limits is GB/T 21005:2007, UV erythema reference action spectrum, standard erythema dose and UV index (China), which defines the daily exposure limit as a flat value of 10 mJ/cm-2 from 250 to 280 nm. Wavelengths below 250 nm are not considered.
The supporting evidence is drawn from the same dataset as CIE Technical Report 187:2010 (recently made freely available online at https://bit.ly/2C7fbnO) and makes similar considerations. CIE 187:2010 concludes the risks associated with erythema and non-melanoma skin cancer from chronic exposure are lower than as reported by the ACGIH.
However, it focuses on risks associated with 254 nm low-pressure mercury lamps in upper-air disinfection systems not intended for direct human exposure. Further, the analysis is not extended to wavelengths below 250 nm. The report provides a valuable contribution to the discussion of proper hazard weighting of UV-C to humans but does not provide conclusive arguments relative to the application of far UV-C.
The disinfection effect imparted by a UV source is dependent on a number of design and experimental factors and can vary substantially as a result. In summary, the fluence required to achieve common disinfection targets often exceeds these limits, and where the limit is higher, it is not by more than an order of magnitude.
Thus, a broad safe disinfection window does not exist, and any application must be carefully reviewed. These standards and legislation were not developed within a risk-based framework to determine a safe dose of UV irradiation for continued human exposure.
Instead, the intent was to define the harm threshold for the maximum unintentional exposure that might occur from incomplete containment of a UV source. They do not recommend the limits for direct exposure of human tissue to UV-C radiation and do not establish protocols for intentional exposure.
Further considerations
Besides the direct interaction of far UV-C radiation with living tissue, other photochemical reactions with clothing, cosmetics, environmental pollution, building materials, etc., should be considered when assessing the suitability of a far UV-C application in populated areas.
The limitation of UV-C study to controlled settings means that comprehensive evaluation of its interactions with human environments has not been undertaken.
The Chapman Cycle (Chapman 1930) describes the competing processes of ozone generation/dissociation from/to molecular oxygen by UV radiation. At its most superficial level, net ozone production occurs in air under UV radiation <242 nm; net ozone dissociation occurs in air under UV radiation >242 nm (Fabian & Dameris 2014).
Therefore, systems designed to apply far UV-C radiation either to or through air will generate some ozone during their operation; however, the risk posed by this generation will depend on the UV power, air flow/stagnation, operation duty cycle, UV spectrum, etc.
Accordingly, risk of ozone exposure should be included in an overall evaluation of safety for far UV-C irradiation in the presence of humans.
If plans to roll out far UV-C technology continue as proposed by its advocates, there will not be sufficient time for the scientific community to reach consensus on its safety. In this case, it may be fully reasonable for members of the public to hold reservations against its application.
Management of the rights of individuals to not be exposed to a technology that is not proven to be safe may pose the greatest challenge to its application.
Conclusion
Far UV-C radiation in the 200 to 225 nm range has been demonstrated as a broadband disinfectant. It is reasonable to assume that far UV-C will be effective in a wide range of disinfection applications for air and surface treatment.
However, more work is required to understand the variation of its efficiency against a wider range of pathogens of interest, and the suitability of each application must be assessed on a case-by-case basis.
Claims of skin and eye safety are supported by early findings in mouse tissues, live mouse models, 3D human tissue models and at chronic exposure levels above those required to achieve a disinfection effect.
To date, these studies have not extended to the exposure of human individuals, and so conclusive evidence on acute and chronic exposure are lacking. Crucially, these works should include considerations of risk factors across a broad range of characteristics such as age, gender, race and medical conditions.
Besides scientific understanding, a better discussion of the core technology is required. Current excimer lamp technology requires optical filtering of the long UV-C wavelength emission spectrum if these are to be used as far UV-C sources for general use. Promotion and sale of unfiltered lamps as “skin safe” by unscrupulous (or uninformed) manufacturers poses a notable risk to public health. The systematic application of high-energy UV radiation across human environments requires greater study into indirect or secondary risk factors, such as interactions with cosmetics, the generation of ozone or other reactive species, and the degradation of materials in the built environment.
There is a burden of proof for overwhelming positive evidence when proposing the introduction of widespread exposure of humans to radiation categorized by the US National Toxicology Program (NTP 2016) as “Reasonably anticipated to be a human carcinogen.” Far UV-C is a promising technology that demands further investigation, though it is the opinion of the IUVA that this burden of proof has not yet been met.
Contact: Rich Simons, [email protected]; Ernest R. Blatchley III, [email protected]; Karl Linden, [email protected]
References
- Beck, S.E., Rodriguez, R.A., Linden, K.G., Hargy, T.M., Larason, T.C., Wright, H.B., (2014) “Wavelength Dependent UV Inactivation and DNA Damage of Adenovirus as Measured by Cell Culture Infectivity and Long Range Quantitative PCR” Environmental Science & Technology 48 (1), pp 591–598. https://doi.org/10.1021/es403850b
- Beck, S.E., Wright, H.B., Hargy, T.M., Larason, T.C., Linden, K.G., (2015) “Action Spectra for Validation of Pathogen Disinfection in Medium-Pressure Ultraviolet (UV) Systems” Water Research, 70:27-37 https://doi.org/10.1016/j.watres.2014.11.028
- Beck, S.E., Hull, N.M., Poepping, C., Linden, K.G., (2018) “Wavelength-Dependent Damage to Adenoviral Proteins Across the Germicidal UV Spectrum” Environ. Sci. Technol. 52, 1, 223–229 https://doi.org/10.1021/acs.est.7b04602
- Brenner, D. (2017) “A new weapon in the fight against superbugs”, Accessed online 2020/06/15: https://www.ted.com/talks/david_brenner_a_new_weapon_in_the_fight_against_superbugs?language=en
- Buonanno, M., Randers-Pehrson, G., Bigelow, A.W., Trivedi, S., Lowy, F.D., Spotnitz, H.M., Hammer, S.M., Brenner, D.J., (2013) 207-nm UV Light – A Promising Tool for Safe Low-Cost Reduction of Surgical Site Infections. I: In Vitro Studies. PLOS ONE 8(10): e76968. https://doi.org/10.1371/journal.pone.0076968
- Buonanno, M., Ponnaiya, B., Welch, D., Stanislauskas, M., Randers-Pehrson, G., Smilenov, L., Lowy, F.D., Owens, D.M., Brenner, D.J., (2017) “Germicidal Efficacy and Mammalian Skin Safety of 222-nm UV Light” Radiation Research 187(4), 493-501. https://doi.org/10.1667/RR0010CC.1
- Buonanno, M., Welch, D., Shuryak, I., Brenner, D.J., (2020) “Far-UVC light (222 nm) efficiently and safely inactivates airborne human coronaviruses.” Sci Rep 10, 10285. https://doi.org/10.1038/s41598-020-67211-2
- Chapman, S., (1930) “A Theory of Upper-Atmospheric Ozone”, Mem. Royal Meteor. Soc., 3(26): 25–103.
- Clauss, M., Springorum A.C., Hartung, J., (2009) “Ultraviolet disinfection with 222 nm wavelength – New options to inactivate UV-resistant pathogens” InSustainable animal husbandry: prevention is better than cure, vol 2.Proc 14th Int Congr Int Soc Anim Hyg (ISAH), Vechta, Germany, 19 to 23July 2009 https://bit.ly/2ZNFAPU
- Fabian, P., Dameris, M. (2014). “Ozone In The Atmosphere – Basic Principles”, Springer, New York. https://doi.org/10.1007/978-3-642-54099-8
- Hull, N.M., Linden, K.G. (2018) “Synergy of MS2 disinfection by sequential exposure to tailored UV wavelengths”. Water Research 143, 292-300 https://doi.org/10.1016/j.watres.2018.06.017
- Harm, W. (1980) “Biological effects of ultraviolet radiation.” Cambridge University Press, Cambridge‐London‐New York‐New Rochelle‐Melbourne‐Sydney
- Jagger, J. (1967) “Introduction to Research in Ultraviolet Photobiology” Prentice-Hall, Inc. Englewood-Cliffs NJ
- Malayeri, A.H., Mohseni, M., Cairns, B., Bolton, J.R., (2016) “Fluence (UV Dose) Required to Achieve Incremental Log Inactivation of Bacteria, Protozoa, Viruses and Algae” IUVA News. 18. 4-6. https://bit.ly/3gvzzOf
- Matafonova, G.G., Batoev, V.B., Astakhova, S.A., Gómez, M., Christofi, N., (2008) “Efficiency of KrCl excilamp (222 nm) for inactivation of bacteria in suspension.” Lett. Appl. Microbiol. 47, 508–513 https://doi.org/10.1111/j.1472-765X.2008.02461.x
- Narita, K., Asano, K., Morimoto, Y., Igarashi, T., Nakane, A., (2018) “Chronic irradiation with 222-nm UVC light induces neither DNA damage nor epidermal lesions in mouse skin, even at high doses” PLoS ONE 13(7): e0201259. https://doi.org/10.1371/journal.pone.0201259
- NTP (National Toxicology Program) (2016) “Report on Carcinogens, Fourteenth Edition”, Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. https://ntp.niehs.nih.gov/go/roc14
- Taylor, W., Camilleri, E., Craft, D.L., Korza, G., Granados, M.R., Peterson, J., Szczpaniak, R., Weller, S.K., Moeller, R., Douki, T., Mok, W.W.K., Setlow, P. (2020) “DNA damage kills bacterial spores and cells exposed to 222-nanometer UV radiation” Appl Environ Microbiol 86:e03039-19. https://doi.org/10.1128/AEM.03039-19
- Vazquez-Bravo, B., Gonçalves, K., Shisler, J.L., Mariñas, B.J., (2018) “Adenovirus Replication Cycle Disruption from Exposure to Polychromatic Ultraviolet Irradiation” Environmental Science & Technology 52 (6), 3652-3659 https://doi.org/10.1021/acs.est.7b06082
- Welch, D., Buonanno, M., Grilj, V. et al. (2018) “Far-UVC light: A new tool to control the spread of airborne-mediated microbial diseases” Sci Rep 8, 2752 https://doi.org/10.1038/s41598-018-21058-w
- Woods, J.A., Evans, A., Forbes, P.D., Coates, P.J., Gardner, J., Valentine, R.M., Ibbotson, S.H., Ferguson, J., Fricker, C., Moseley, H., (2015) “The effect of 222‐nm UVC on healthy volunteer skin” Photodermatol. Photoimmunol. Photomed., 31: 159-166. https://doi.org/10.1111/phpp.12156
- Yamano, N., Kunisada, N., Kaidzu, S., Sugihara, K., Nishiaki-Sawada, Aiko., Ohashi, H., Yoshioka, A., Igarashi, T., Ohira, A., Tanito, M., Nishigori, C., (2020) “Long‐term Effects of 222‐nm ultraviolet radiation C Sterilizing Lamps on Mice Susceptible to Ultraviolet Radiation”. Photochem Photobiol. https://doi.org/10.1111/php.13269




